Mycena lamprocephala C. B. Soares & J. S. Oliveira, 2024
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.634.3.1 |
|
DOI |
https://doi.org/10.5281/zenodo.10556404 |
|
persistent identifier |
https://treatment.plazi.org/id/007F352F-FFCA-626A-FF24-75336955B1EE |
|
treatment provided by |
Plazi |
|
scientific name |
Mycena lamprocephala C. B. Soares & J. S. Oliveira |
| status |
sp. nov. |
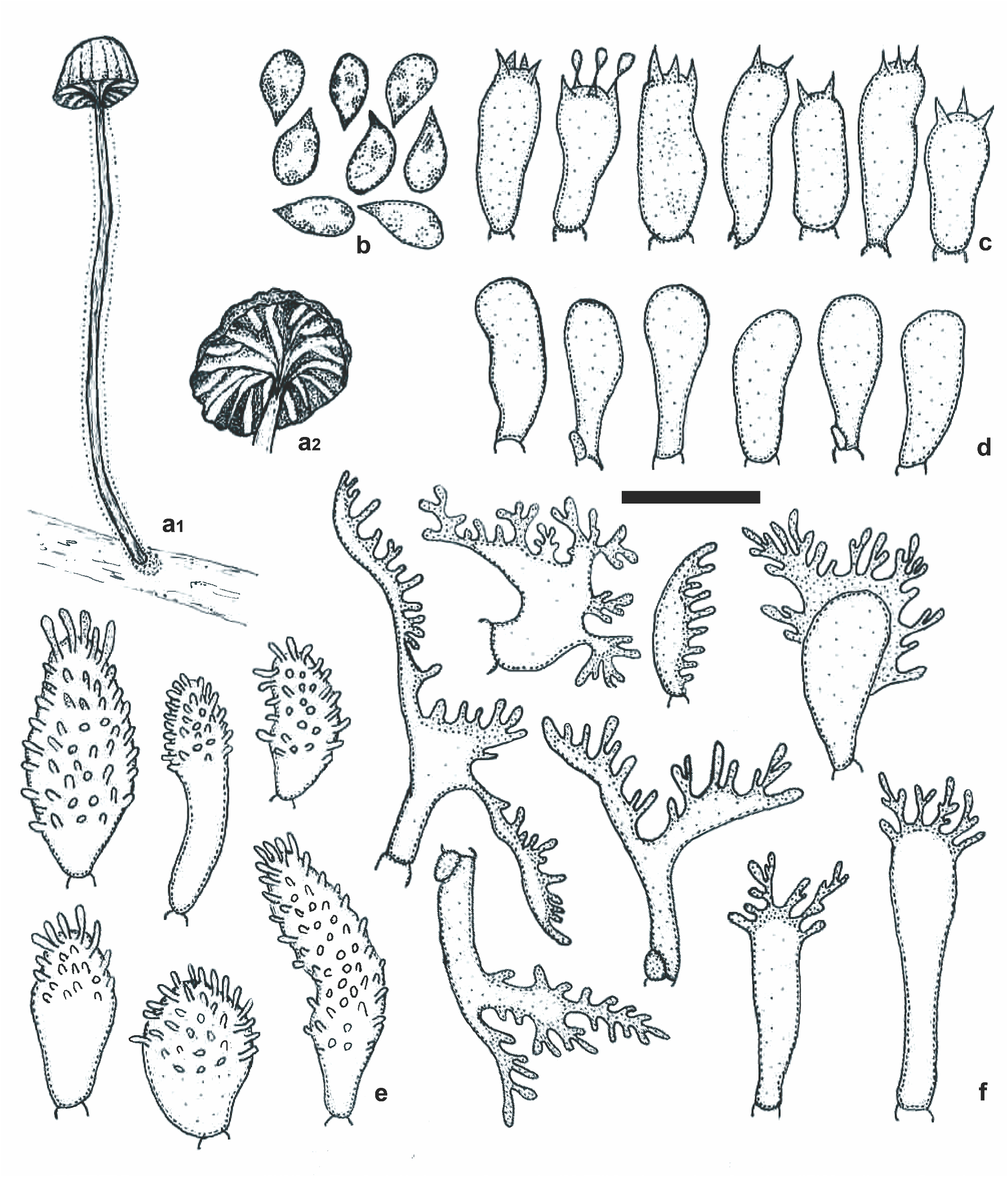
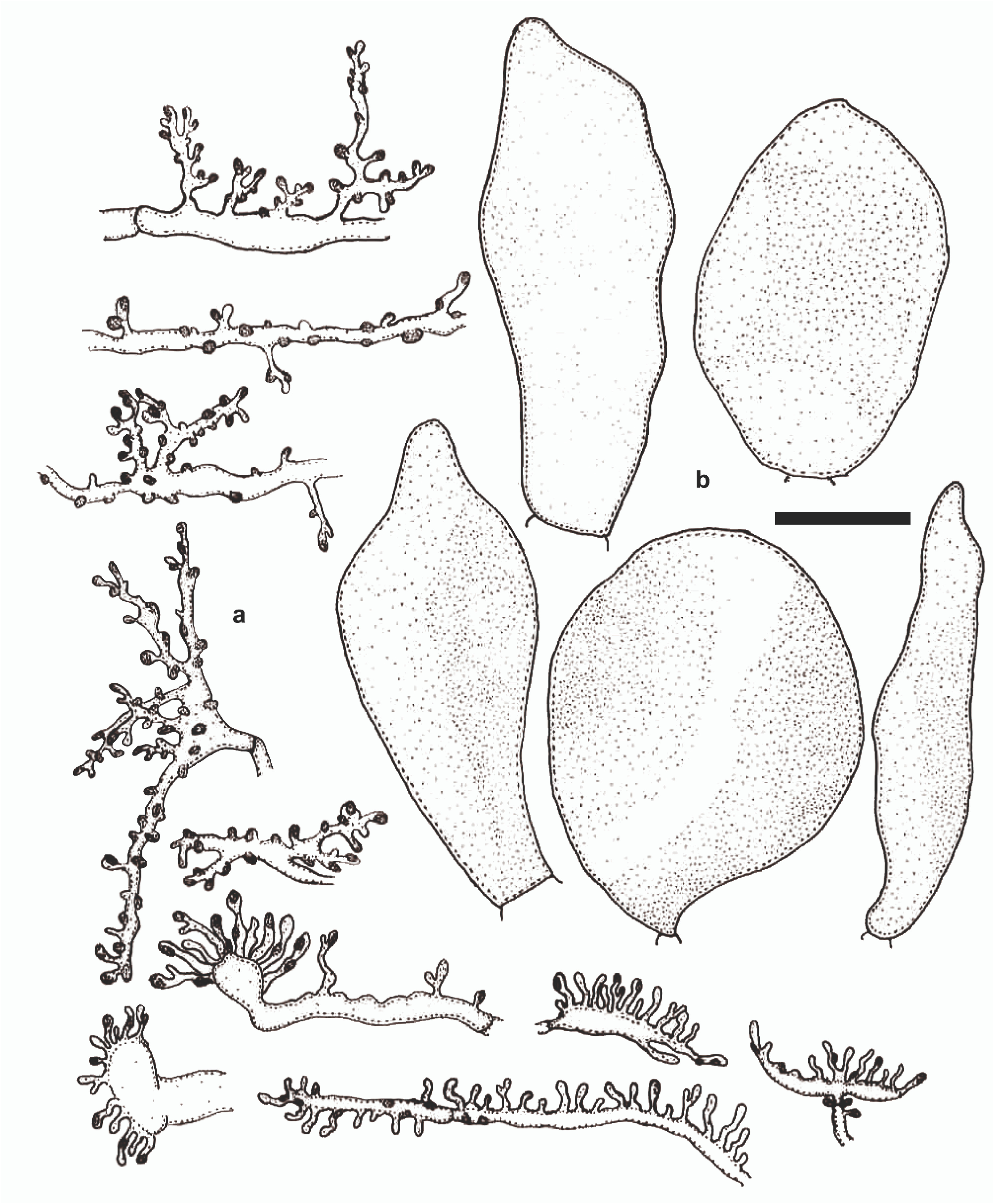
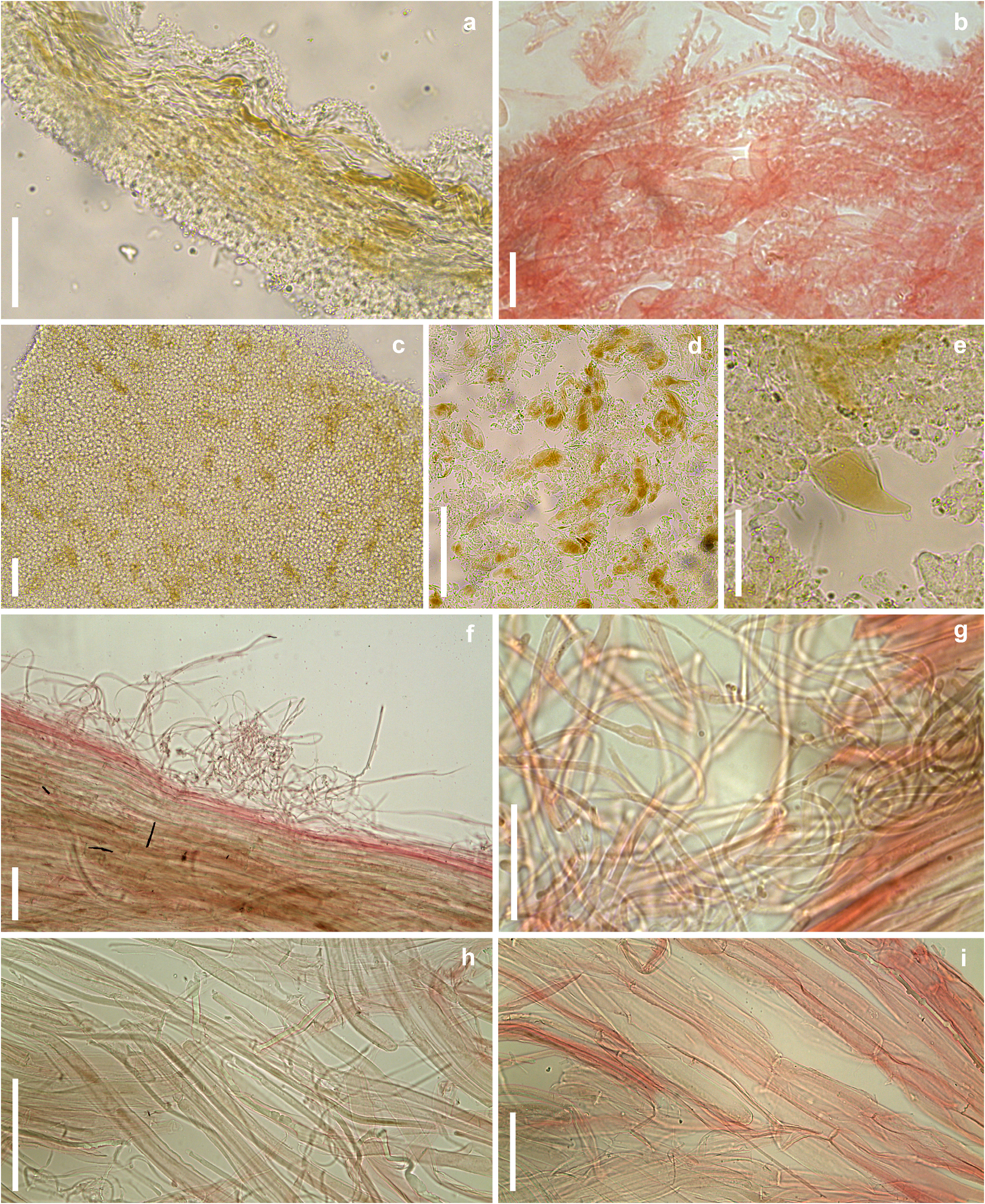
Mycena lamprocephala C. B. Soares & J. S. Oliveira , sp. nov. Figs. 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 .
MycoBank MB 850761
Holotype:— BRAZIL. AMAZONAS State: Municipality of Manaus, upper Cuieiras River Reserve — INPA, access trail to the base, 2°42’44.6”S 60°23’17.5”W, solitary, in dried eudicotyledonous leaves and sticks in the litter, terra-firme forest, 30 May 2019, J.S. Cardoso; T. Morbach & F. S. Andriolli JS801 ( INPA 292235 View Materials ). GenBank: ITS = OR727532. GoogleMaps
Etymology:—From the Greek: λαμπρόσ (lamprós) = brighting, and κεφάλι (kefáli) = head; refers to the luminescent pileus.
Diagnosis: Pileus (5–6 mm diam.) olivaceous brown, luminescent; stipe glutinous; pileus and lamellae trama with brown, cystidioid hyphae segments; pleurocystidia clavate to subfusiform, densely spinulose; cheilocystidia branched, ramose; pileipellis composed of diverticulate-coralloid hyphae immersed in a gelatinous matrix. The combination of these characters differentiate it from any similar species.
Description:— Basidiome omphalinoid, thin, small. Pileus 5–6 mm diam., hemispherical to convex-truncate, becoming convex, orbicular, sulcato-striate, with shallow vein-like grooves between sulci, center depressed, margin decurved, edge crenate to crenulate, slightly wavy, surface glabrous at the center or when moist, finely furfuraceous to pruinose at the margin when dry, slightly rugose, waxy; light brown (7D6) to brown (7E7) with a slight olive tinge, center and sulci dark brown (7F6); context gray-brown (7E3), very thin (<1 mm). Lamellae subdecurrent to decurrent, arcuate to curved, or slightly sinuate, distant, L = 11, lamellulae ventricose, l = 1, faces pruinose, whitish brown with a slight olive tinge (5B3), edges paler, white to cream (5A2), hymenium between the lamellae concolorous with the context. Stipe 33–39 × 0.8 mm, centrally attached, cylindrical, thin, equal, smooth, hollow, fragile, longitudinally ridged, covered with a thick hyaline gelatinous layer, gray-brown (7E3) mixed with olive brown tinge, or concolorous with the pileus, subinsititious with a small basal disc formed by rigid gelatinous material, orange-brown (5C4), without basal mycelium. Luminescence of the basidiome restricted to the pileus, glow is a green sheen, sometimes intermittent, flashing at a slow frequency; scanty mycelial luminescence on the substrate also noticed.
Basidiospores 5.4–8.3 (9) × (2.4) 3–5.2 μm [x rm = 6.9–8.1 × 4–4.5 μm, x mm = 7.5 (± 0.8) × 4.2 (± 0.5) μm, Q rm = 1.8– 1.9, Q mm = 1.8 (± 0.3), n/s = 30, s = 3], ellipsoid to subellipsoid, some lacrymoid, hyaline, smooth, thin-walled, amyloid. Basidia 17.4–25 × 6.1–7.9 μm, clavate, smooth, hyaline or with fuscous content, 2–4 sterigmate, thin-walled, inamyloid, with or without clamp connection at the base. Basidioles 12.8–21.3 × 4.9–7 μm, cylindrical to clavate, smooth, hyaline, thin-walled, inamyloid, with clamp connection at base. Pleurocystidia 19.3–35.1(56.2) × 6.2–15.4(23.1) μm, clavate to subfusiform, cylindrical-clavate, sometimes pyriform, thin-walled, hyaline, densely spinulose at the apex, base smooth or almost so, spinulae 1.3–5.7 × 0.4–1.6 μm, cylindrical to verruciform, dense, longer at apex of the main body, scarce and smaller towards the base, clamp connections not observed. Cheilocystidia abundant, hyaline, composing the sterile lamellar edge, 16.5–44.3 × 2.9–7.2 μm, clavate, coralloid, irregular in shape, bilobed to branched, ramose, thin-walled, inamyloid, clamp connection present at base; diverticula, cylindrical, digitiform, 2.3–4.6 × 0.1–1.7 μm, simple to furcate, hyaline, thin-walled. Lamellar trama dextrinoid, irregular, interwoven hyphae, cylindrical, 3–4.8 μm diam., regular in outline, smooth, thin-walled, clamp connections present. Pileus trama dextrinoid, subregular, composed of cylindrical hyphae, 1.5–6.6 μm diam., smooth, thin-walled, hyaline, clamp connections absent. Lamellae and pileus trama mottled with brown, cystidioid hyphal segments imbedded in the regular hyphal trama; cystidioid segments 22.5–82.7 × 10.9–26.1 μm, clavate, obclavate, lageniform to fusiform, inflated, with brown pigmentation (6E6), irregular in outline, smooth, thin-walled. Pileipellis an ixocutis of repent, interwoven, cylindrical to irregular in outline, branched, diverticulate hyphae, 1.9–2.7 µm diam., hyaline, thin-walled, inamyloid, imbedded in a gelatinous matrix; diverticula verruciform to irregularly cylindrical, 0.6–1.9 × 0.4–0.9 μm, simple or furcate, hyaline, thin-walled. Stipe trama strongly dextrinoid, cortical hyphae subregular, cylindrical, 6,4–17 μm diam., regular in outline, smooth, thin-walled, clamp connections not observed; internal hyphae with a complex arrangement, parallel, regular in outline and in the arrangement, with diverse shapes, narrow and filamentous, and cylindrical to catenulated, inflated, 6.1–20.6 µm diam., hyaline, smooth, thin-walled, clamp connections absent; cystidioid segments also present. Stipetipellis composed of slender hyphae, 1.5–4.3 μm diam., filiform, smooth, cylindrical, occasionally forming tufts or tangles in some points on the surface of the stipe, absent in other places, thin-walled, clamp connections not observed; no gelatinous layer or matrix after treatment in KOH solution. Basal disc formed by interwoven hyphae similar to those on the cortex of the stipe, some inflated hyphae with dense cellular content, 6.6–15.7 µm diam., thick-walled, 1.4–4.2 µm diam., smooth, without clamp connections.
Growth habit:—Solitary, growing on dry leaves of eudicotyledonous plant, on the midrib, leaf blade and on decomposing branches in the litter, in the Amazon rainforest, in terra-firme forest type, between May and June.
Additional material examined:— BRAZIL. Amazonas, Municipality of Manaus, upper Cuieiras River Reserve — INPA, access trail to the base, 2°42’44.6”S 60°23’17.5’’W, 29 May 2019, J.S. Cardoso GoogleMaps ; T. Morbach & F. S. Andriolli JS800 ( INPA 292234 View Materials , GenBank : ITS = OR727531, LSU = OR762048 ) ; Pajurá trail, 2°42’43.1”S 60°23’18.9’’W, 04 June 2019, D.L. Komura GoogleMaps ; T. Morbach ; S.S. Vieira; A. Santos de Paula & E.S. Amorim DLK2704 ( INPA 292236 View Materials , GenBank : ITS = OR727533, LSU = OR762049 ) .
Notes:— Mycena lamprocephala is typically characterized by a brownish basidiome, thick glutinous stipe, pleurocystidia and cheilocystidia of dissimilar shape, and a pileipellis composed of diverticulate-coralloid hyphal layer immersed in a gelatinous matrix.
The great diversity of morphological structures combined, especially microscopic, strongly distinguish M. lamprocephala from any other known mycenoid species and the set of characteristics is not clearly congruent with any section currently recognized in the genus. Mycena aspratilis Maas Geesteranus & de Meijer (1997: 44) ( Mycena sect. Aspratiles Maas Geesteranus & de Meijer (1997: 44) ), in which luminescence was not verified, seems to be the most similar species in morphology. It differs by having a stipe with a white pubescent basal disc due to the presence of thick-walled, cylindrical caulocystidia, cylindrical, thick-walled cheilocystidia, densely covered by digitiform diverticula, and reduced pleurocystidia similar to the cheilocystidia ( Maas Geesteranus & de Meijer 1997). Another somewhat similar species, M. lacrimans Singer (suggestively akin to Mycena sect. Aspratiles ,) although luminescent, diverges in having a whitish pileus, dry stipe, lack of pleurocystidia, differently shaped cheilocystidia with broad, knoblike, apical appendages, and distinct pileipellis elements ( Singer 1989, Desjardin & Braga-Neto 2007). Luminescence was reported in the pileus, lamellae and the stipe of M. lacrimans ( Desjardin & Braga-Neto 2007) but not from the mycelium in the substrate as in M. lamprocephala ( Fig. 1d View FIGURE 1 ).
Mycena lamprocephala may be comparable to species of Mycena sect. Euspeireae , but species of that section differ by having a gelatinous separable pellicle forming the pileipellis and by the more or less smooth hymenial cystidia ( Maas Geesteranus & de Meijer 1997). Also, in species of sect. Euspeireae, the cortical hyphae of the stipe can be observed immersed in a gelatinous matter in KOH solution while this was not seen in M. lamprocephala because, although probably an ixocutis when fresh, the gelatinous matter dissolves completely in KOH solution.
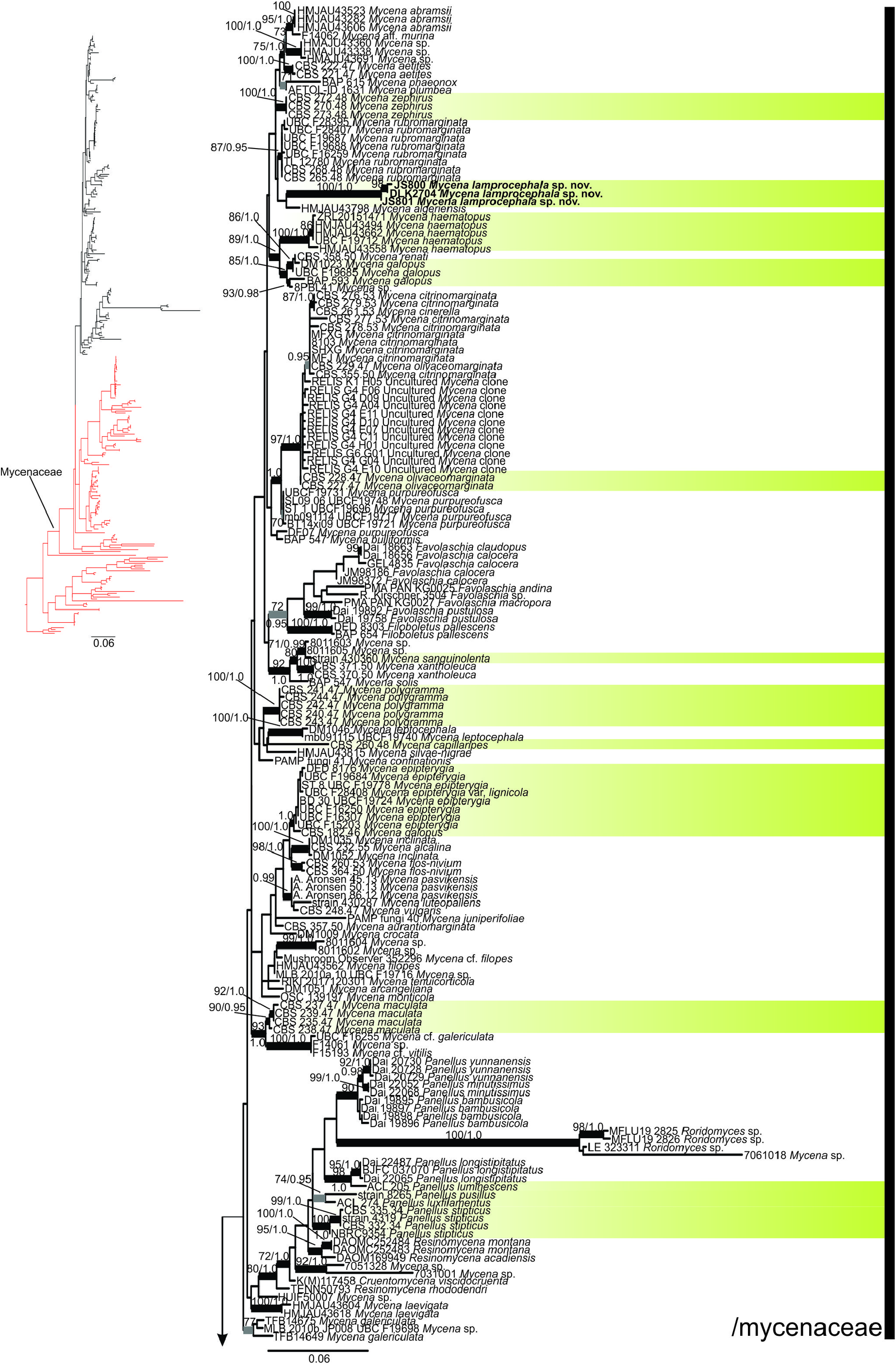
Phylogenetic analysis
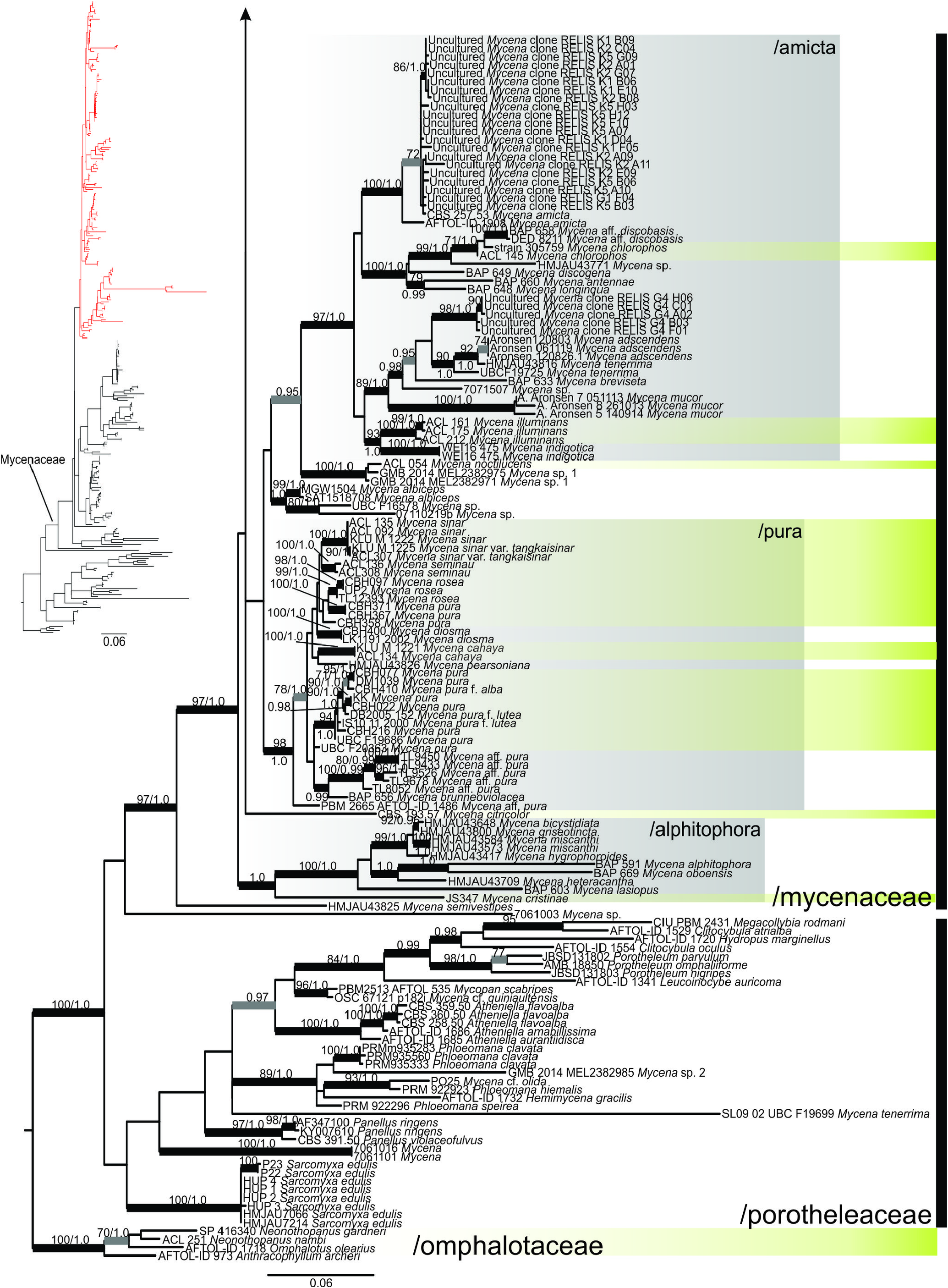
The resulting ML tree is displayed in two parts in Figs. 5 View FIGURE 5 and 6 View FIGURE 6 , with quite similar topology to the BA tree. Three major clades are represented: /omphalotaceae as the outgroup which also has luminescent species, /porotheleaceae having genera segregated from Mycena along with some luminescent species, and /mycenaceae which is the largest and more well-represented clade in this analysis. Clade /mycenaceae is strongly supported (BS 97; PP 1.0) while /porotheleaceae is unsupported. Within /mycenaceae, M. lamprocephala , depicted in green-yellow, is placed in the upper part of the clade ( Fig. 5 View FIGURE 5 ). This part is more unresolved with multiple branches into polytomy, having members of Cruentomycena R.H. Petersen, Kovalenko & O.V. Morozova (2008: 123) Panellus , Resinomycena Redhead & Singer (1981: 151) and Roridomyces forming a more distinctive cluster, Favolaschia and Filoboletus in another cluster, and several Mycena lineages scattered in many branches without intermediate to deep resolution. Mycena lamprocephala seems to be sister to M. algeriensis Maire ( Kühner 1938: 490) but without support, and these two are sister to M. rubromarginata (Fr.) P. Kummer (1871: 109) without support. The second part of /mycenaceae ( Fig. 6 View FIGURE 6 ) is more resolved than the first part, harboring at least three strongly supported clades and two small species branches. Strongly supported small clades or branches in /porotheleaceae represent the genera Atheniella , Clitocybula , Hemimycena Singer (1938: 194) , Hydropus Kühner ex Singer (1948: 127) , Leucoinocybe , Megacollybia Kotlába & Pouzar (1972: 220) Mycopan Redhead, Moncalvo & Vilgalys (2013: 1) , Phloeomana , Porotheleum Fries (1818: 272) and Sarcomyxa P. Karsten (1891: 62) while some named Mycena and Panellus are just embedded. Relationships among these lineages are partly unsupported.
The BLAST searches (Jul. 2023) with the ITS and the LSU sequences of M. lamprocephala did not yield significant results as the species is genetically very distant from all species represented in GenBank.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |