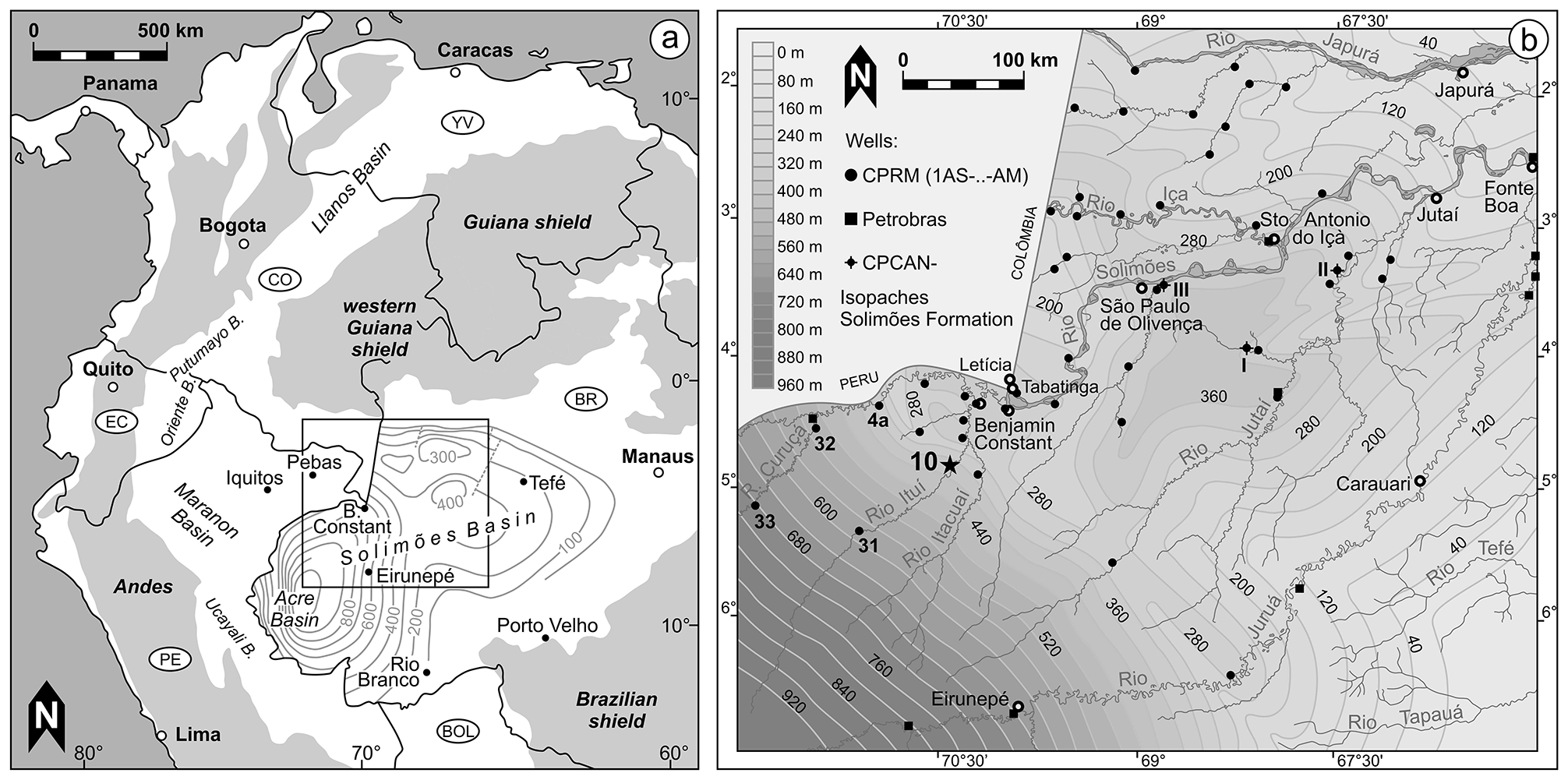
Cyprideis sulcosigmoidalis ( Purper, 1979 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3899.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D78F2010-08E1-45C0-86FF-7F2D3601070D |
|
persistent identifier |
https://treatment.plazi.org/id/017587FE-FFB3-FFE1-71F4-DE73FD9BFE12 |
|
treatment provided by |
Felipe |
|
scientific name |
Cyprideis sulcosigmoidalis ( Purper, 1979 ) |
| status |
|
Cyprideis sulcosigmoidalis ( Purper, 1979)
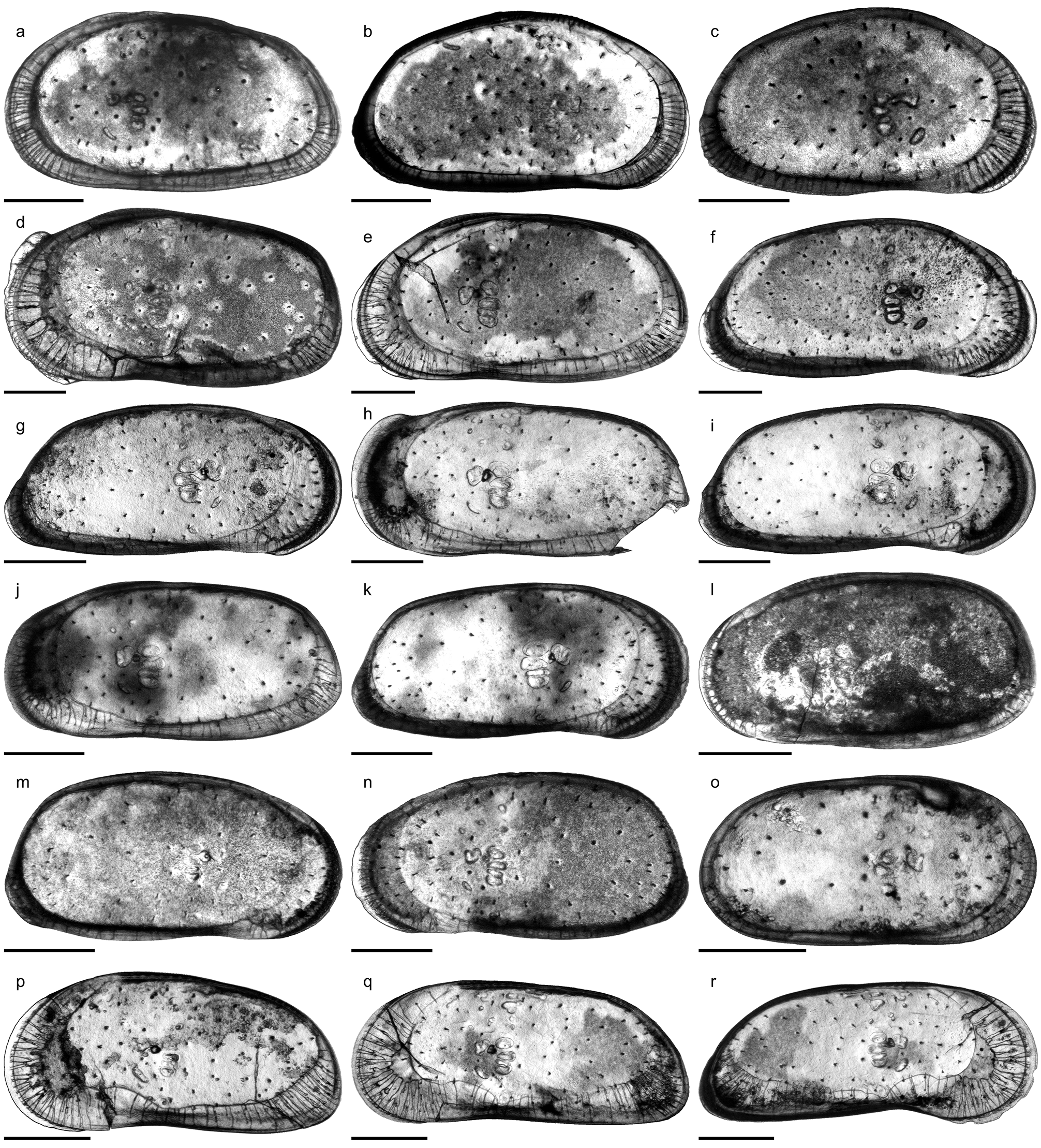
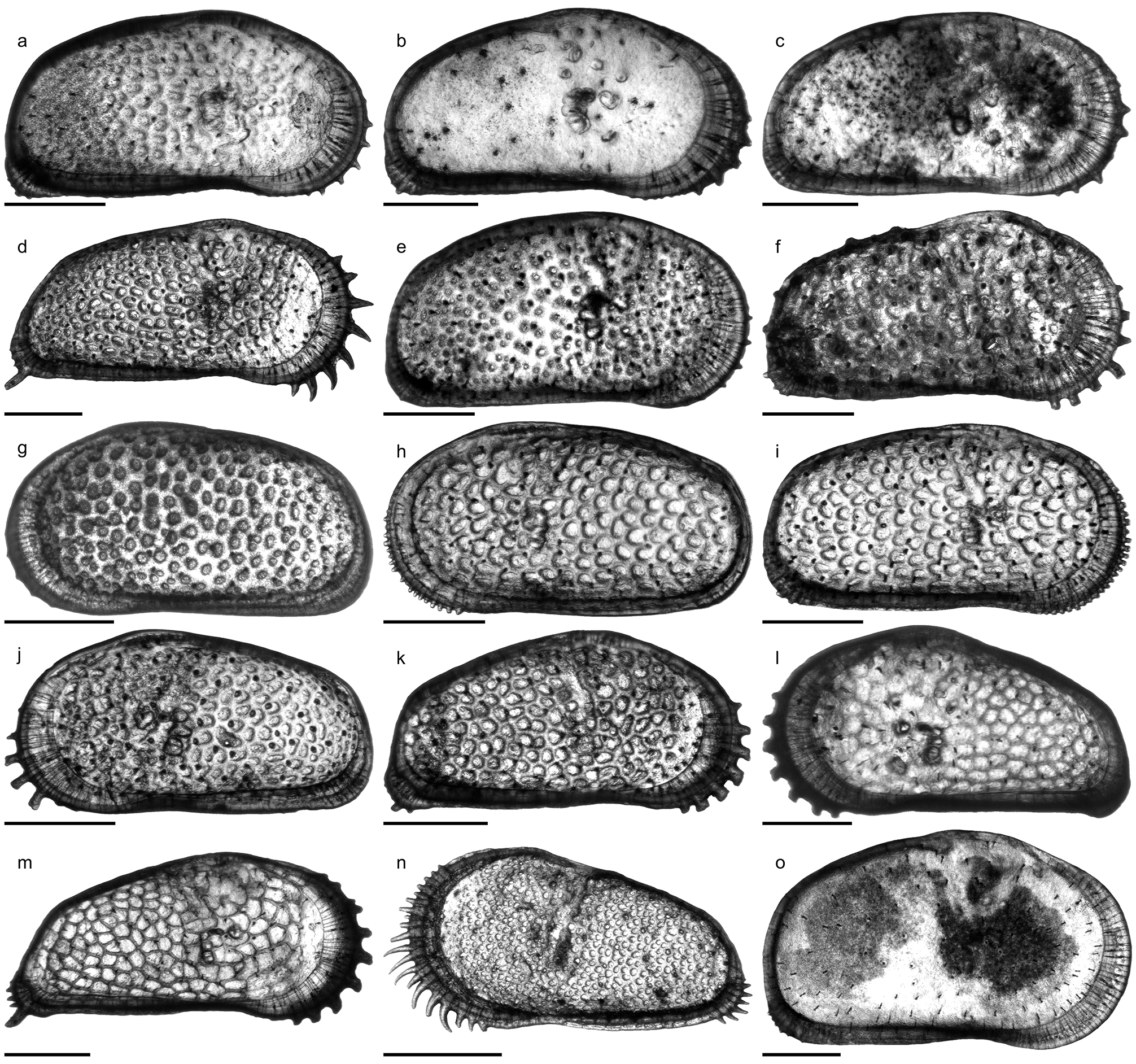
Fig. 6o View FIGURE 6 ; Pl. 12, Figs. 20–34; Pl. 13, Figs. 1 View FIGURE 1 –21; Pl. 14, Figs. 1 View FIGURE 1 –21
1977 Cytheridea sp. nov. A—Purper: 361; Pl. 2, Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 .
* 1979 Cytheridea sulcosigmoidalis Purper , sp. nov. —Purper: 226–227; Pl. 1, Figs. 11–18.
pars 1980 Cyprideis purperi purperi subsp. nov. —Sheppard & Bate: 99–101; Pl. 7, Fig. 13 and probably Fig. 12. [non Text-fig. 2; Pl. 7, Figs. 1 View FIGURE 1 –11; Pl. 8, Figs. 1–2 View FIGURE 1 View FIGURE 2 ]
1998 Cyprideis sulcosigmoidalis ( Purper, 1979) —Whatley et al.: 236; Text-fig. 2; Pl. 3, Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 .
1998 Cyprideis sp. 4 —Whatley et al.: 237; Pl. 3, Figs. 16–20.
1998 Cyprideis aulakos sp. nov. —Muñoz-Torres et al.: 94; Text-fig. 2; Pl. 2, Figs. 7 View FIGURE 7 –11.
1998 Cyprideis sulcosigmoidalis ( Purper, 1979) —Muñoz-Torres et al.: 100; Pl. 4, Figs. 16–18.
1998 Cyprideis sp. aff. C. retrobispinosa Purper and Pinto —Swain: 3; Pl. 6, Figs. 1–8 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 .
2011 Cyprideis aulakos —Linhares et al.: 95; Figs. 3 View FIGURE 3 /3–4.
2011 Cyprideis sulcosigmoidalis —Linhares et al.: 95; Figs. 4 View FIGURE 4 /5–6.
Material. 1,839 valves; samples AM 10/3, 6–7, 15–16, 19–31, 33, 35, 39–44.
Dimensions (total range over all samples). R ♀ l = 0.84–1.16 (0.99), h = 0.50–0.68 (0.58; n = 186); L ♀ l = 0.92–1.17 (1.04), h = 0.57–0.72 (0.63; n = 14); R ♂ l = 0.98–1.13 (1.08), h = 0.51–0.66 (0.60; n = 15); L ♂ l = 0.96–1.22 (1.08), h = 0.57–0.73 (0.63; n = 13).
Remarks. The current material complies with C. sulcosigmoidalis of Purper (1979) and Linhares et al. (2011) and C. aulakos of Muñoz-Torres et al. (1998 = Cyprideis sp. 4 in Whatley et al. 1998; Linhares et al. 2011). The specimen figured by Sheppard & Bate (1980) on plate 7, figure 13 (and most probably figure 12; Purper & Pinto 1985) as well as Cyprideis sp. aff. C. retrobispinosa in Swain (1998) actually belong to C. sulcosigmoidalis .
C. sulcosigmoidalis is a subovate–subtrapezoidal, punctate, avestibulate species with pronounced, sinuous sulcus. While the ventral margin of left valves is only slightly concave, the anteroventral margin is more curved in right valves, which cause a more pronounced ventral concavity. Puncta are randomly arranged centrally but form concentric rows along the free valve margin. The hinge (right valve) is formed by robustly denticulated anterior and posterior elements; the long, crenulated median element is composed of a short anteromedian groove and a long posteromedian bar. Marginal pore canals are numerous, simple or rarely bifurcated; normal pores are of sievetype. Males are more elongated and have a more oblique posterior margin than females ( Purper 1979; Muñoz- Torres et al. 1998; Whatley et al. 1998; note: due to its outline, the “female” holotype represents in fact a male individual). Anterior (both valves) and posterior denticles (only right valves) as illustrated by Muñoz-Torres et al. (1998) and Whatley et al. (1998) have not been mentioned in the original description by Purper (1979). Conversely, Purper (1979: 226) stated for C. sulcosigmoidalis : “it does not present spines on the anterior margin”.
By examining the original descriptions and figurations of C. sulcosigmoidalis and C. aulakos , there is virtually no clear-cut difference between both taxa (neither in outline, development of the sulcus, the inner lamella, pore canals nor in the hinge). In addition to the trait of marginal denticulation, which was later introduced by Muñoz- Torres et al. (1998) and Whatley et al. (1998), the only difference is that C. aulakos “lacks parallel punctuation peripherally and the ornament is smoother […] although some specimens are densely punctate anteriorly” ( Whatley et al. 1998: 237; see also Muñoz-Torres et al. 1998: 94).
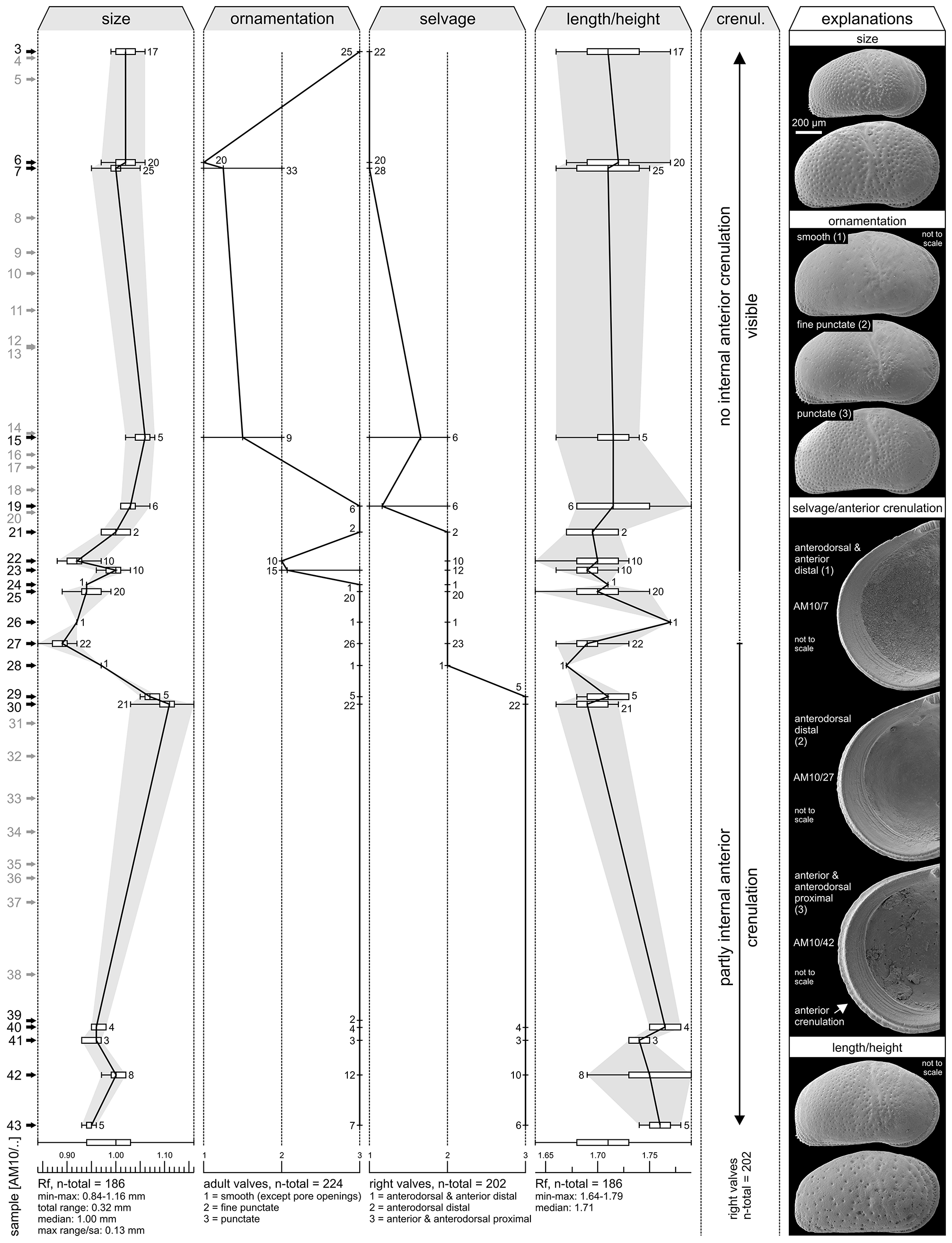
Within the present material considerable variations in ornamentation within samples (e.g. AM10/23: Pl. 13, Figs. 8 View FIGURE 8 , 11) and, especially, between samples are evident (e.g. AM10/7: Pl. 12, Fig. 27; AM10/3: Pl. 12, Fig. 21). Specimens from the lower part of the core (AM10/43–25) and the uppermost sample (AM10/3) have a sulcosigmoidalis - type ornament. In-between, transitional morphotypes occur (AM10/23–15) and only in samples AM10/7–6 smooth, aulakos - type valves dominate ( Fig. 7 View FIGURE 7 ). Based on this observation, the diagnostic feature “ornament” sensu Muñoz-Torres et al. (1998) is problematic and most likely ecologically controlled.
Anterior marginal denticles are only poorly developed in our specimens (rather present as a crenulation; frequently only seen in internal view; Fig. 7 View FIGURE 7 ). As far as the material enables, anterior denticulations are restricted to the lower part of 1AS-10-AM (~up to AM10/27). However, referring to the original description of C. sulcosigmoidalis , in this case the degree of marginal denticulation seems to be a weak diagnostic trait. After personal observation (M.G.), it can be stated that in core 1AS-33-AM (sample depth: 290.1 and 356.3 m), C. sulcosigmoidalis with numerous anteromarginal denticles on right valves occur in the strata where Cyprideis caraionae was described by Purper & Pinto (1985). The ornamentation (almost smooth to coarsely punctate) varies considerably within these samples, analogous to the material of 1AS-10-AM. Conversely, C. sulcosigmoidalis from the uppermost layers (sample depth: 37.5 and 44.1 m) of core 1AS-4a-AM lacks these denticles but equals the specimens from AM10/ 30 in outline, ornament and, in particular, in the position of the selvage (see below). Possible evolutionary trends (e.g. progressive reduction of spines) require further investigations.
For these reasons, we suppose that ornament and marginal denticulation are not sufficient characters to delineate C. aulakos from C. sulcosigmoidalis and regard them as synonyms.
Throughout the core no significant variations of the inner lamella, pore canals, hinge or muscle scars were found. Nonetheless, C. sulcosigmoidalis varies noticeably in size in core 1AS-10-AM (e.g. large valves in AM10/ 30, small ones in AM10/27; Fig. 7 View FIGURE 7 ), but this is not straightforwardly correlated with the characters discussed above and probably linked to another ecological parameter.
A further observation concerns the length/height ratio ( Fig. 7 View FIGURE 7 ): specimens of the lowermost samples (AM10/ 43–39) are more elongated in outline than the specimens up-section (e.g. Pl. 14, Figs. 18–21). Because of the limited and badly preserved material from this part of the core, it remains highly speculative to discuss a possible phylogenetic trend here.
Nevertheless, one—admittedly subtle—character seems to change gradually within the core section: the course of the selvage on the anterior margin of right valves ( Fig. 7 View FIGURE 7 ) and, connected with this (but less well expressed), the course of the anterodorsal outline. In specimens from samples up to AM10/29, the selvage is located proximally in the anterior and anterodorsal valve region. It shifts distally in the anterodorsal region in specimens from samples AM10/28-15, while in specimens from the uppermost samples AM10/7-3, the selvage is placed distally in both the anterior and anterodorsal valve regions. Potentially, this shift of the selvage marks a delicate evolutionary change within C. sulcosigmoidalis , which could be of further biostratigraphic importance. Additional analyses of other cores are necessary to prove or refute this claim.
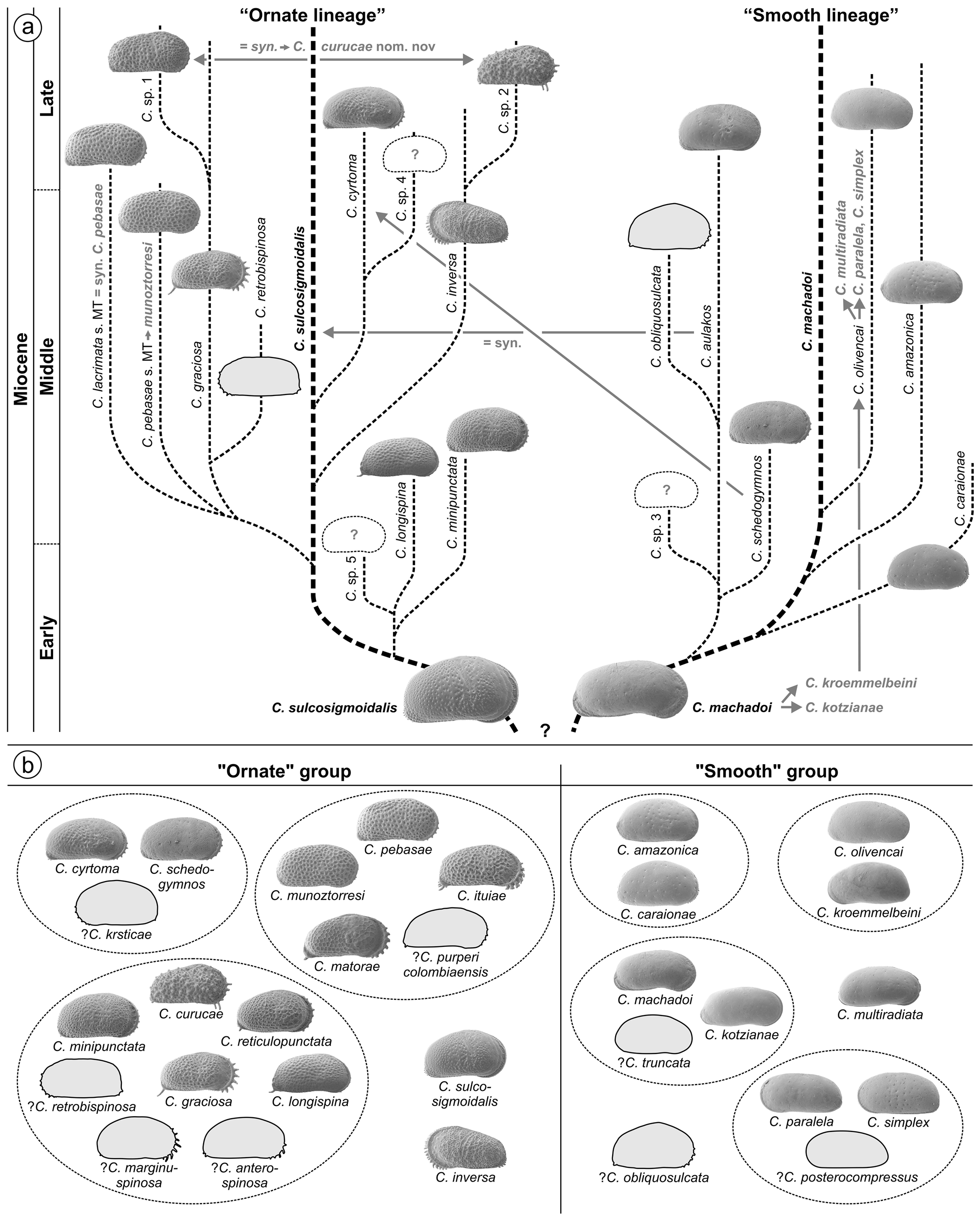
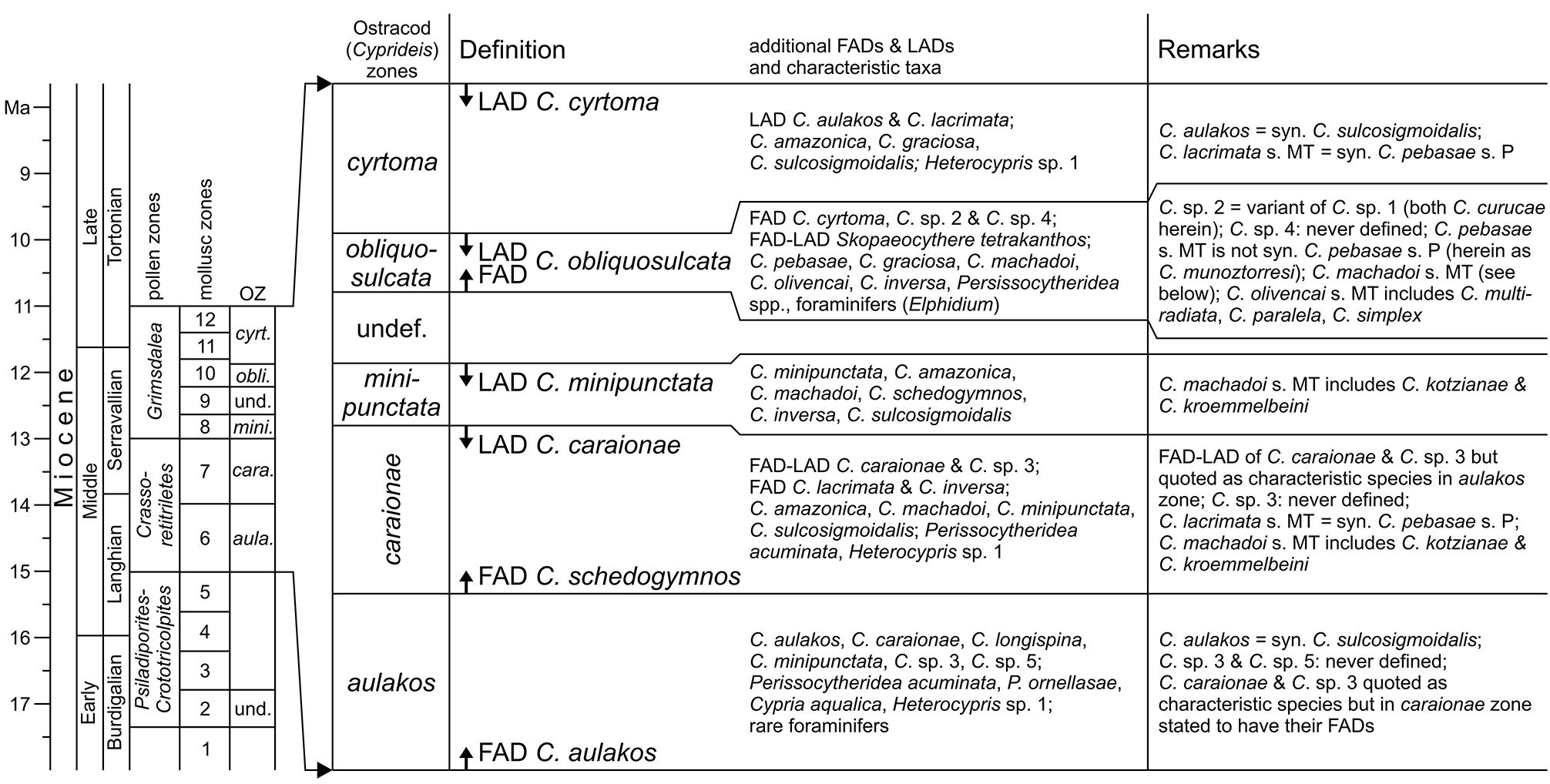
However, C. sulcosigmoidalis and C. aulakos are placed in two different lineages ( C. sulcosigmoidalis in the “ornate”, C. aulakos in the “smooth” lineage) in the phylogenetic scheme of Whatley et al. (1998) and Muñoz- Torres et al. (2006). Following our observations, both are not only very closely related but are synonyms. Thus, the proposed phylogeny of Amazonian Cyprideis needs substantial reorganisation ( Fig. 3a View FIGURE 3 ). Furthermore, the “first appearance datum” of C. aulakos marks the lower boundary of the C. aulakos zone ( Muñoz-Torres et al. 2006). With the rejection of this species, this zone is largely challenged ( Fig. 8 View FIGURE 8 ). It is left open to further investigations, if the occurrence of the aulakos -morphotype has an ecological implication and is at least of (local) ecostratigraphic value.
Occurrence (including the synonymous C. aulakos ). Western Amazonia ( Brazil, Colombia, Peru), early Middle to early Late Miocene ( C. aulakos – C. cyrtoma zone; Muñoz-Torres et al. 2006; chronostratigraphic correlation after Wesselingh & Ramos 2010).
5. Discussion
5.1. Biostratigraphic implications
Muñoz-Torres et al. (2006) proposed an ostracod-based biozonation for the Pebas/Solimões Formation, which is —similar to mollusc zones ( Wesselingh et al. 2006b)— tightly linked to earlier palynological zonations ( Hoorn 1993, 1994b; Fig. 8 View FIGURE 8 ). Intensive debates about definitions of pollen zones and their chronostratigraphic allocation still characterise significant uncertainties of western Amazonia’s stratigraphy ( Jaramillo et al. 2010; Latrubesse et al. 2010; Silva-Caminha et al. 2010; Dino et al. 2012; Gross et al. 2013; compare Jaramillo et al. 2011 for detailed palynological zonations of the Llanos Basin, Colombia). Here, we apply the stratigraphic concept of Wesselingh & Ramos (2010; correlation of mollusc and ostracod zones; chronostratigraphy) as well as of Wesselingh et al. (2006b; correlation of pollen and mollusc zones; Fig. 9 View FIGURE 9 ), being aware that considerable readjustments of biozone correlations and chronology are required.
Among the ostracod index species sensu Muñoz-Torres et al. (2006) found in core 1AS-10-AM, C. minipunctata has its last occurrence in sample AM10/31 ( Fig. 10 View FIGURE 10 ). As the last appearance of this species defines the top of the C. minipunctata zone ( Muñoz-Torres et al. 2006), the productive samples below AM10/30 (> 141.2 m depth) can be assigned to the C. minipunctata zone and/or to the downward succeeding C. caraionae zone. C. schedogymnos is restricted to samples AM10/ 40–44 in the present core. It points to the same direction, because it appears in the C. caraionae zone and vanishes at the end of the C. minipunctata zone ( Muñoz-Torres et al. 2006). Similarly, C. simplex (as far as it is differentiated as a separate species) occurs in the C. caraionae zone. The latter zone is characterised by the first and last appearance of C. caraionae , which is, however, missing in the current materials. Unpublished palynological evaluations (M. Ebner, Tübingen) of core 1AS-10-AM recorded the pollen index taxon Grimsdalea magnaclavata Germeraad, Hopping & Muller, 1968 throughout the core (down to 336 m depth, sample AM10/48, altitude: - 251 m). The onset of the Grimsdalea zone coincides with the lower boundary of the C. minipunctata zone as suggested by Wesselingh & Ramos (2010). Based on this, and supported by the absence of C. caraionae , we assign the core interval from AM10/31–44 (> 141.2 to 218.1 m depth) to the C. minipunctata zone.
Up-section (AM10/30), C. cyrtoma and C. curucae (= Cyprideis sp. 1 and 2 in Muñoz-Torres et al. 2006) occur for the first time in our record. Both species have their first appearance at the base of the C. obliquosulcata zone ( Muñoz-Torres et al. 2006). This ostracod zone is characterised by the first and last appearance of C. obliquosulcata , which has not been identified here. Accordingly, the core proportion between AM10/30 and AM10/3 can be attributed to the C. obliquosulcata and the following C. cyrtoma zone only, and a differentiation of both zones is tentative.
Muñoz-Torres et al. (2006) mentioned that the minute limnocytherid Skopaeocythere tetrakanthos Whatley, Muñoz-Torres & Van Harten, 2000 is restricted to the C. obliquosulcata zone and the presence of the foraminifer Elphidium is also characteristic for this interval. S. tetrakanthos has been found in AM10/22, associated with dwarfed elphidiid foraminifers (also in AM10/23; pers. observ., M.G.). Thus, the boundary between the C. obliquosulcata and C. cyrtoma zones rests questionably somewhere above AM10/22 (<116.4 m depth).
5.2. Remarks to the western Amazonian Cyprideis species flock
The appraisal of western Amazonian Cyprideis basically depends on two works: the initial monograph by Purper (1979) and the publication of Whatley et al. (1998).
Purper (1979) discovered the endemism of this fauna, leading to the erection of several new species and genera (later extended by Sheppard & Bate 1980; Purper & Pinto 1983, 1985; Purper & Ornellas 1991). These studies provided 26 formally described “ Cyprideis ” species, placed in nine different genera (seven are endemic for western Amazonia).
Conversely, Whatley et al. (1998; and complementarily Muñoz-Torres et al. 1998) applied a “broader” concept—on species and genus level alike (a discussion of the latter is beyond the scope of the present contribution). Seven earlier established species were considered as synonyms, five new species were added and nine species of Sheppard & Bate (1980), Purper & Pinto (1983, 1985), Purper & Ornellas (1991) were not treated. With the emendation of the generic diagnosis of Cyprideis ( Whatley et al. 1998) , all these species were transferred to that genus, hence comprising 24 (inclusively synonyms: 31) formally described Amazonian Cyprideis species.
Here, we encounter 20 Cyprideis species. Five species, placed together by Whatley et al. (1998), are revalidated (one additional remains unclear), two species of these authors are synonymized, and two new species are defined. Thus, 30 species now form the Miocene Amazonian Cyprideis inventory ( Fig. 3 View FIGURE 3 ).
Although each separate valve character can be subject to serious intraspecific variation in Cyprideis , detailed observations on a considerable number of valves (~16 % out of ~7,200 counted valves (ESM 1, 2) revealed sufficient traits for species delineations (discussion in 4.2.). In particular, between samples, valves’ sizes and ornaments of species repeatedly vary and are obviously environmentally controlled (a discussion of potential ecological influxes is the subject of upcoming works). Nevertheless, within samples (fossil populations), size and “basic” patterns of ornamentation of species form valuable discriminating traits. Similarly, variations in marginal denticulation of specimens between strata occur, but their principal expression (e.g. shape, position) provides a valuable diagnostic character, frequently already perceptible in juvenile stages. By application of the generic conception of Whatley et al. (1998), the development of the inner lamella and of marginal pore canals also offer an effective taxonomic tool for species differentiation. In addition, hinge structures (inclusively reversed hinges) contribute to species diagnoses.
The observed intraspecific plasticity (especially between samples) necessitates an extensive photographic illustration. We are aware that this work is just a further step to register Cyprideis ’ radiation in western Amazonia. Future studies, focussing on selected species only, may render some species, as outlined here, to be species complexes or lineages.
Whatley et al. (1998) and Muñoz-Torres et al. (2006) proposed a hypothetical phylogeny for Amazonian Cyprideis through Miocene times, which speculatively originates from one or two ancestor(s), giving rise to a “smooth” and an “ornate” lineage. Limited by the comparably short time interval covered by well 1AS-10-AM (? <2 Ma), we do not attempt to redesign that phylogeny here. Nonetheless, solely based on comparative morphology a further characterisation of the “smooth” and “ornate” groups was possible, accompanied by a reorganisation of species relations and (sub-)groupings, respectively (see 4.3.–4.5.). These taxonomic adjustments substantially concern Cyprideis ’ phylogeny and biozonations as well.
The current study underlines once more Cyprideis ’ remarkable capability to produce species flocks. Western Amazonian Cyprideis comply with the criteria of a species flock ( Lecointre et al. 2013; see also Schön & Martens 2004; Sturmbauer 2008):
i) endemicity: up to now not a single species has been recorded in adjacent areas;
ii) monophyly: to date this criterion is hardly verifiable, and probably Amazonian Cyprideis is not monophyletic in a strict sense. Perhaps Purper’s original generic subdivision reflects the situation more properly; in any case, several closely related, quite rapidly evolving species (or species complexes) are proved;
iii) speciosity: due to the present study, 30 formally described Cyprideis species exist in western Amazonia; additionally, several other species are recorded in the literature, although left in open nomenclature until now. This strongly hints to a much higher, still unrecorded species richness within this vast and little explored region;
iv) ecological diversity: this criterion remains difficult to attest due to limited research and the fact of dealing with extinct taxa; based on rare sedimentologic cross-references, ecological diversity within a highly structured wetland is possible ( Gross et al. 2013); the current results demonstrate the sympatric occurrence of up to 12 Cyprideis species , which may indicate adaptations to different microhabitats;
v) habitat dominance: regularly, Cyprideis holds more than 90 % in western Amazonian ostracod assemblages during the Early and Middle Miocene ( Muñoz-Torres et al. 1998; Whatley et al. 1998; and this study), however, it decreases to c. 36 % close to its disappearance in Late Miocene times ( Ramos 2006; Gross et al. 2013).
6. Conclusions
The micropalaeontologic investigation of well 1AS-10-AM (Solimões Basin; Brazil) permits a review of about 2/3 of hitherto described Miocene Cyprideis species from western Amazonia. More than 7,000 valves (out of estimated ~12,000) were counted, of which>1,000 were subject to detailed examination (light and SEM photography; basic morphometrics). This allows for conclusions on the taxonomic value of observable valve characters as well as for a demonstration of intraspecific variability (as far as possible including sexual and ontogenetic polymorphism).
Based on our observations, we refine existing Cyprideis species definitions (5 species are revalidated, 2 synonymised, 2 renamed and 2 defined) as well as earlier proposed species (sub-)groupings. Due to the occurrence of some ostracod index species, the core comprises sediments of the C. minipunctata to C. cyrtoma ostracod zones, corresponding to the Grimsdalea pollen zone and a late Middle to early Late Miocene age.
We regard the current study as a base for upcoming, more advanced analyses (e.g. geometric morphometric approaches or geochemical analyses) as well as a further step in illuminating the amazing Cyprideis species flock of western Amazonia’s geological past.
| AM |
Australian Museum |
| R |
Departamento de Geologia, Universidad de Chile |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.