Pseudophlepsius binotatus ( Signoret, 1880 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5270.3.8 |
|
publication LSID |
lsid:zoobank.org:pub:F05A4310-0B0B-46A5-8EC0-72FEC9F52410 |
|
DOI |
https://doi.org/10.5281/zenodo.7863591 |
|
persistent identifier |
https://treatment.plazi.org/id/021487A0-D458-753D-4FAC-DA77FEA7F88B |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudophlepsius binotatus ( Signoret, 1880 ) |
| status |
|
Pseudophlepsius binotatus ( Signoret, 1880) View in CoL View at ENA
( Figs. 8–17 View FIGURES 1–17 , 45–71 View FIGURES 41–55 View FIGURES 56–83 )
Phlepsius comma Haupt, 1917: 243–245 View in CoL (synonymy by Haupt, 1929)
Phlepsopsius liupanshanensis Li, 2011: 172–173 View in CoL (synonymy by Cao, Xing, 2022)
P. binotatus pseudalhageos Zachvatkin, 1953: 235
P. binotatus septentrionalis Zachvatkin, 1953: 235 View in CoL
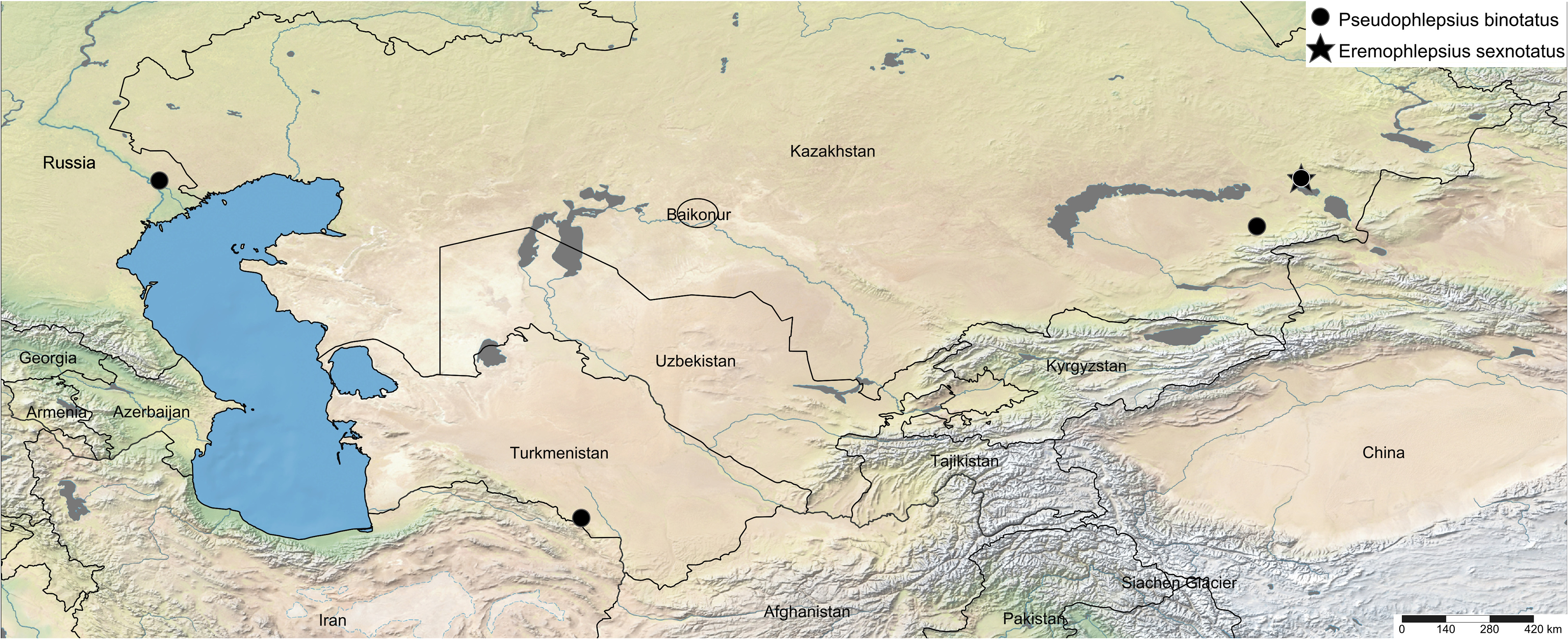
Material examined. Many specimens from northeastern Iran, southern Turkmenistan, Uzbekistan, Kyrgyzstan, Kazakhstan, and the Lower Volga Region of Russia were studied.
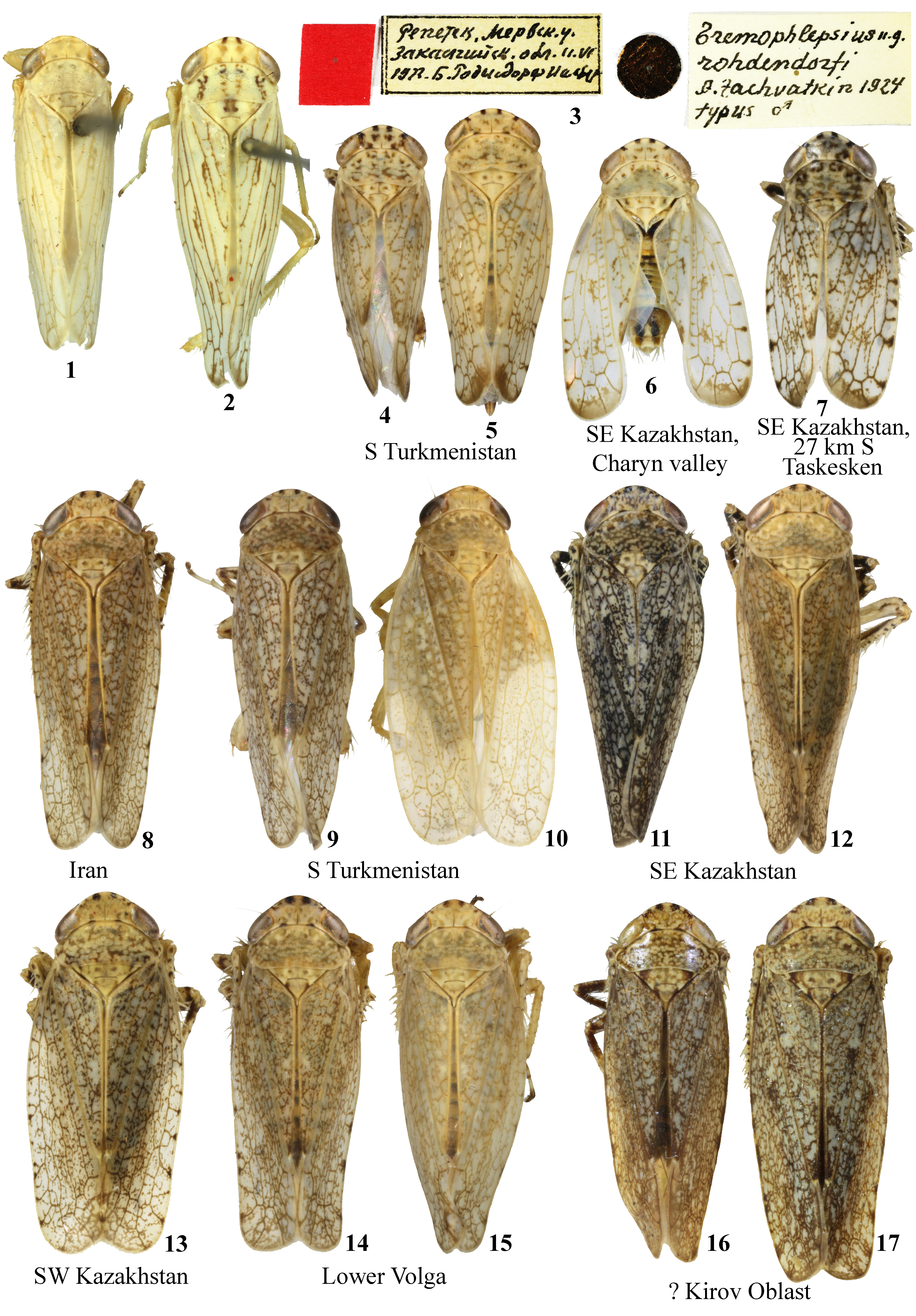
Description. Pale yellowish with whitish forewings. Head, pro-, and mesonotum with numerous small dark spots; head also with two larger black spots on fore margin next to midline. Forewings with brown veins and with dense fine line or speckled pattern ( Figs. 8–17 View FIGURES 1–17 ). Intensity of dark pigmentation varies greatly even between specimens from same sample ( Figs. 9–10, 11–12 View FIGURES 1–17 ).
Aedeagus U-shaped, stems wide, with denticles in distal halves on ventral side ( Figs. 56–67 View FIGURES 56–83 ). Stem apices broadly or narrowly rounded (for example, Figs. 59, 62–63 View FIGURES 56–83 ), sometimes wide angular and obliquely cut ( Figs. 58, 64, 66–67 View FIGURES 56–83 ); specimens with different penis shape can be found in the same sample ( Figs. 56–59 View FIGURES 56–83 ). Basal processes narrower than in Eremophlepsius , curved inwards, with hook-like tips. Position of processes in relation to penis stems varies, which is visible in lateral view ( Figs. 57a–62a, 64a–67a View FIGURES 56–83 ). Styles with long narrow tips evenly bent outward ( Fig. 68 View FIGURES 56–83 ). Valve large, subgenital plates without macrosetae, with elongated narrow tips. Pygofer lobes with rather narrow, rounded apices and pointed, almost straight, directed slightly dorsally ventral processes ( Figs. 69–70 View FIGURES 56–83 ).
Body length: ♁, 4.6–5.4 mm; ♀, 5.7–6.4 mm.
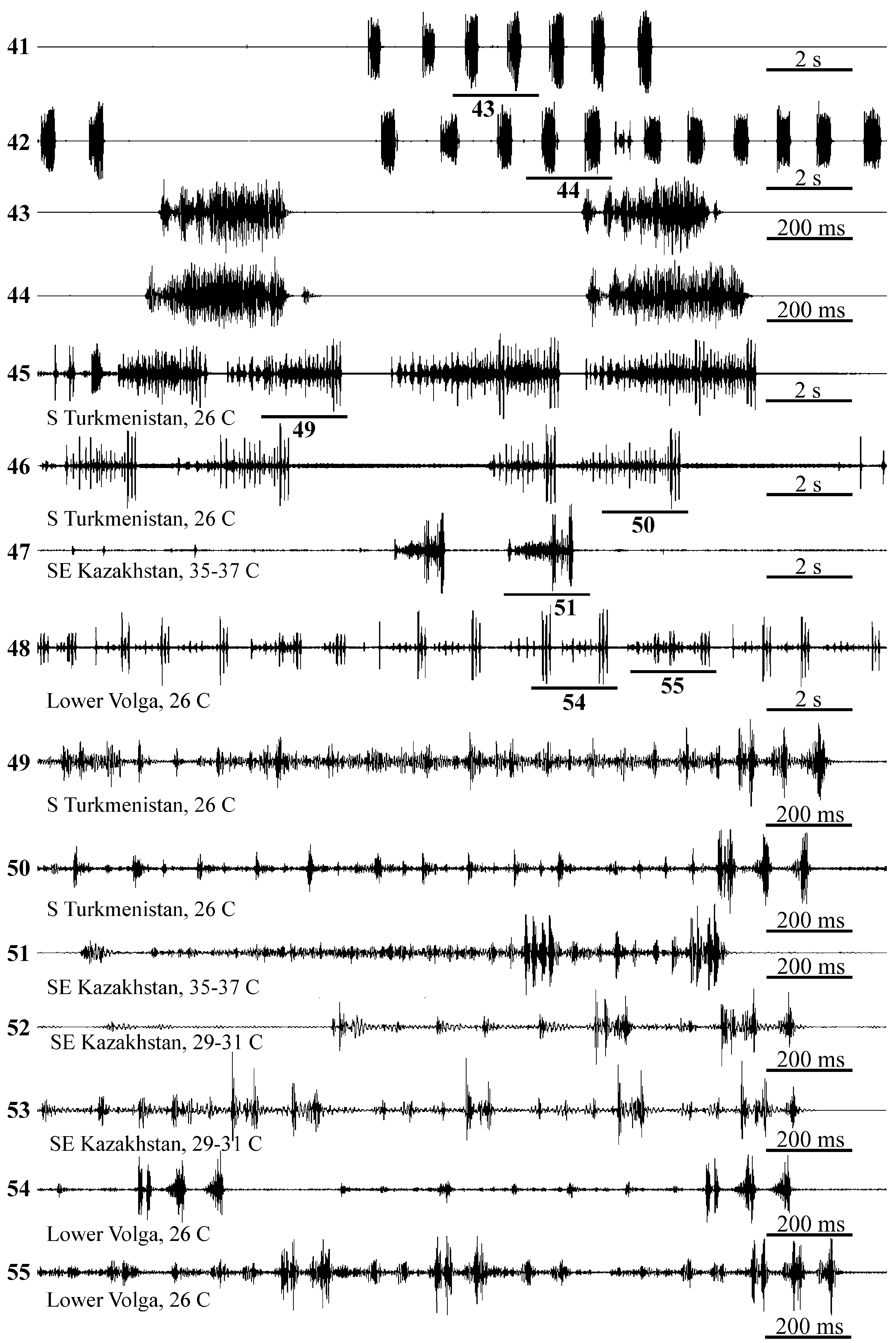
Male calling signal. Signals of males from four localities were investigated ( Fig. 40 View FIGURE 40 ).
1. Southern Turkmenistan, Dushak village, ca. 170 km SE of Ashkhabad, from Alhagi persarum in the desert, 19. V. 1990, signals of two males recorded at 26 oC .
2. Southeastern Kazakhstan, environs of Aksu village, 20 km N of Zhasagurov, from A. pseudalhagi near the river, 27. VI. 2019, signals of two males recorded at 29–31 oC .
3. Southeastern Kazakhstan, Urzharsky Region, 27 km south of Taskesken, A. pseudalhagi in the steppe on the riverbank, 24. VI. 2022, signals of three males recorded at 35–37 oC .
4. Russia, Lower Volga Region, Dosang Railway Station , ca. 60 km N of Astrakhan, from A. pseudalhagi in the desert, 2. VII. 2000, signals of three males recorded at 26 oC .
Calling signal is a phrase lasting from 1–1.5 up to 4 s in our recordings ( Figs. 45–48 View FIGURES 41–55 ); phrase duration can vary between the males from the same locality ( Figs. 45–46 View FIGURES 41–55 ). Phrase begins with a variable train of pulses with lower amplitude and ends with a high-amplitude syllable usually consisting of four pulses ( Figs. 49–55 View FIGURES 41–55 ). Sometimes, one or several high-amplitude syllables also present in the middle part of a phrase ( Figs. 51–53, 55 View FIGURES 41–55 ). The ratio of the amplitudes of the initial part of the phrase and the final syllable also varies in males from the same locality ( Figs. 54–55 View FIGURES 41–55 ). As in other insects, pulse repetition period decreases with increasing temperature (cf. Figs. 51 and 52 View FIGURES 41–55 ).
Distribution. Morocco, Turkey, Moldova, Ukraine, southern European Russia, Transcaucasia, and Central Asia ( Mityaev, 2002, 2015; Cao & Xing, 2022), eastwards as far as southern Siberia (Tyva; Vilbaste, 1980), Mongolia ( Emelyanov, 1977), and China (Ningxia; Li et al., 2011). Rare species in the steppes of Eastern Europe, common in the semideserts and deserts of the Lower Volga Region and Central Asia.
Remarks. Since this species is highly variable, some authors divided it into several subspecies and even into two species.
According to Signoret (1880), the description of P. binotatus is based on the material from Sarepta (presently, the southern part of Volgograd) and Persia; it should be noted that old entomological materials with labels “Sarepta” in fact could have been collected in different localities throughout Volgograd and Astrakhan Oblasts. Signoret (1880) does not list type material and, accordingly, gave no indications whether Sarepta or Persia should be considered a type locality.
Phlepsius comma was described from Kerki, southeastern Turkmenistan ( Haupt, 1917) and later synonymized under Pseudophlepsius binotatus by the same author ( Haupt, 1929).
Zachvatkin (1953) believes that the taxon from the Lower Volga Region should be considered nominotypical and considers P. binotatus binotatus from the Lower Volga and P. binotatus comma from Central Asia to be different subspecies. Dubovsky (1966) shares his opinion, but raises the Asian taxon to the species rank and use for it the name P. comma .
Comparison of specimens from Iran and the Lower Volga Region and of signal recordings from southern Turkmenistan and Astrakhan Oblast did not reveal any constant differences between East European and Asian populations and supported the synonymy established by Haupt (1929). Moreover, we did not find any differences supporting subspecific status of these taxa.
In addition, Zachvatkin (1953) described two subspecies based on small differences in body proportions and coloration.
Specimens of P. binotatus pseudalhageos from semidesert Mil Plain in Azerbaijan are absent in the collection of the ZMMU.According to the primary description, this taxon differs from other subspecies in coloration, the male body index and the so-called forewing index (the ratio of its length to width). The male body indices of P. binotatus pseudalhageos (3.39–3.46) and P. binotatus binotatus (3.45–3.56) slightly overlaps, but the forewing indices are different (3.24–3.40 and 3.10–3.15). Like specimens from the Lower Volga Region and Central Asia, P. binotatus pseudalhageos was collected from Alhagi . Zachvatkin (1953) suggests that P. binotatus pseudalhageos may also occur in northwestern Iran. In this regard, it would be very important to compare it with the Asian P. binotatus comma , but these data are absent in the article. Also, the maximum body width and, as a consequence, body index strongly depend on the position of the forewings, which can be seen by comparing, for example, Figs. 9 and 10 View FIGURES 1–17 or 11 and 12, but Zachvatkin does not describe how it was measured.
P. binotatus septentrionalis was described based on one male and two females from Kirov Oblast ( eastern European Russia), where it was found on well-lit forest clearings. P. binotatus septentrionalis differs from P. binotatus binotatus by darker coloration and the male body index (3.73 vs. 3.45–3.56) but in females these indices overlap (3.48–3.57 and 3.25–3.51, respectively). Since Alhagi spp. are not distributed that far north, this subspecies certainly feeds on other plants. In the collection of ZMMU there are one male and two females of P. binotatus with rather dark coloration, with labels “clearing in pine-deciduous forest” without geographical data ( Figs. 16–17 View FIGURES 1–17 , 67–67a View FIGURES 56–83 ). Apparently, these are type specimens, since such a habitat is not usual for this species, and it is unlikely that the collection will include specimens from exactly the same biotope, but from another region .
As can be seen on Figs. 9–12 and 14–15 View FIGURES 1–17 , light and dark colored specimens can be found in the same population. For this reason, coloration cannot be used for separation of subspecific taxa in this species. Description of new subspecies based on morphometric differences between small samples from geographically distant localities is incorrect because of the lack of material (in particular, in the case of P. binotatus septentrionalis ) and because the absence of specimens from intermediate localities does not allow us to understand whether these samples are real subspecies or only different variants of clinal variability. Apparently, this incorrectness is attributable to the unfinished nature of Zachvatkin’s manuscript, because it was found in his archive and published after his death.
Our data confirm that P. binotatus is heterogeneous in coloration and the aedeagus shape; however, it is impossible to identify clear boundaries between different populations from Alhagi spp. that would allow dividing it into subspecies. For clarification the status of P. binotatus septentrionalis , investigation of populations from other hosts is necessary.
| ZMMU |
Zoological Museum, Moscow Lomonosov State University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pseudophlepsius binotatus ( Signoret, 1880 )
| Tishechkin, Dmitri Yu. 2023 |
Phlepsopsius liupanshanensis
| Li, Z. Z. & Dai, R. H. & Xing, J. C. 2011: 173 |
P. binotatus pseudalhageos
| Zachvatkin, A. A. 1953: 235 |
P. binotatus septentrionalis
| Zachvatkin, A. A. 1953: 235 |
Phlepsius comma
| Haupt, H. 1917: 245 |