Minagrion veredae, Vilela & Jacques & Souza, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5374.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:E9F72C3C-A005-415D-8D36-2C0EA9036C26 |
|
DOI |
https://doi.org/10.5281/zenodo.10248447 |
|
persistent identifier |
https://treatment.plazi.org/id/03812674-FFA2-FF9C-7CC0-D017FDE5F868 |
|
treatment provided by |
Plazi |
|
scientific name |
Minagrion veredae |
| status |
sp. nov. |
Minagrion veredae sp. nov. Vilela & Souza
( Figs. 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 )
Holotype. ♂, BRAZIL ( Z329 ), Minas Gerais State, Grande Sertão Veredas National Park , 15.iv.2023, (15°6’ S, 45°48’ W, 660 to 900m asl), P.S. Vital leg., IFSULDEMINAS. GoogleMaps
Paratypes. Same data as holotype, except: 1♂ ( Z326 ), E.D.F. Ferreira leg., IFSULDEMINAS; GoogleMaps 2♂♂ ( Z112 , Z140 ), 05.xi.2022, L. Millani leg., IFSULDEMINAS; GoogleMaps 2♂♂ ( Z195 , Z198 ), 28.i.2023, T.P. Gouvêa leg., IFSULDEMINAS; GoogleMaps 1♂ ( Z313 ), M.M. Souza leg., FAAL. GoogleMaps
Etymology. Named veredae (name in genitive case) in honor to the palm swamp environments, called veredas in Brazil, which harbors vast biodiversity and is currently being threatened by human activities. This name is also a reference to the type locality in Minas Gerais state, the Grande Sertão Veredas National Park, who is named after the veredas, but also inspired by the homonymous book, written by the “Mineiro” João Guimarães Rosa (1908–1967).
Head ( Fig. 1 View FIGURE 1 ). Dorsally black; postocular spots light blue, elongated, merging with the occipital bar, of same color; frons angulated; antefrons, postfrons, labrum and mandibles greenish; clypeus and postclypeus black; ventral face of the head grey or bluish adjacent to the eye, remainder black.
Thorax ( Fig. 1 View FIGURE 1 ). Anterior lobe of prothorax blue anteriorly, remainder black; medial lobe black dorsally with circular blue lateral spots; posterior lobe entire, with a medial convexity, black with blue on the lateral margins. Pterothorax black dorsally with metallic copper reflections; lower half of mesepisternum with a blue stripe; mesepimeron black, with a posterior blue spot; mesinfraepisternum black with a small pale yellow/green area; anterior portion of metepisternum pale yellow, remainder blue; remainder of pterothorax pale yellow/green.
Legs ( Fig. 1 View FIGURE 1 ). Pale yellow; femoral armature black, spines decreasing in size towards the apex; tibiae pale yellow, spines subequal in size; tarsal apex and claws with dark apex, the supplementary tooth on tarsal claw well developed.
Wings ( Fig. 1 View FIGURE 1 ). Hyaline, pterostigma dark brown, with pale contours, 1 cell long; 9 Px in HW, 10 Px in FW; CuA extending for 7 cells distal to vein descending from subnodus in FW, 8 in HW; MP reaching wing posterior margin in FW and HW.
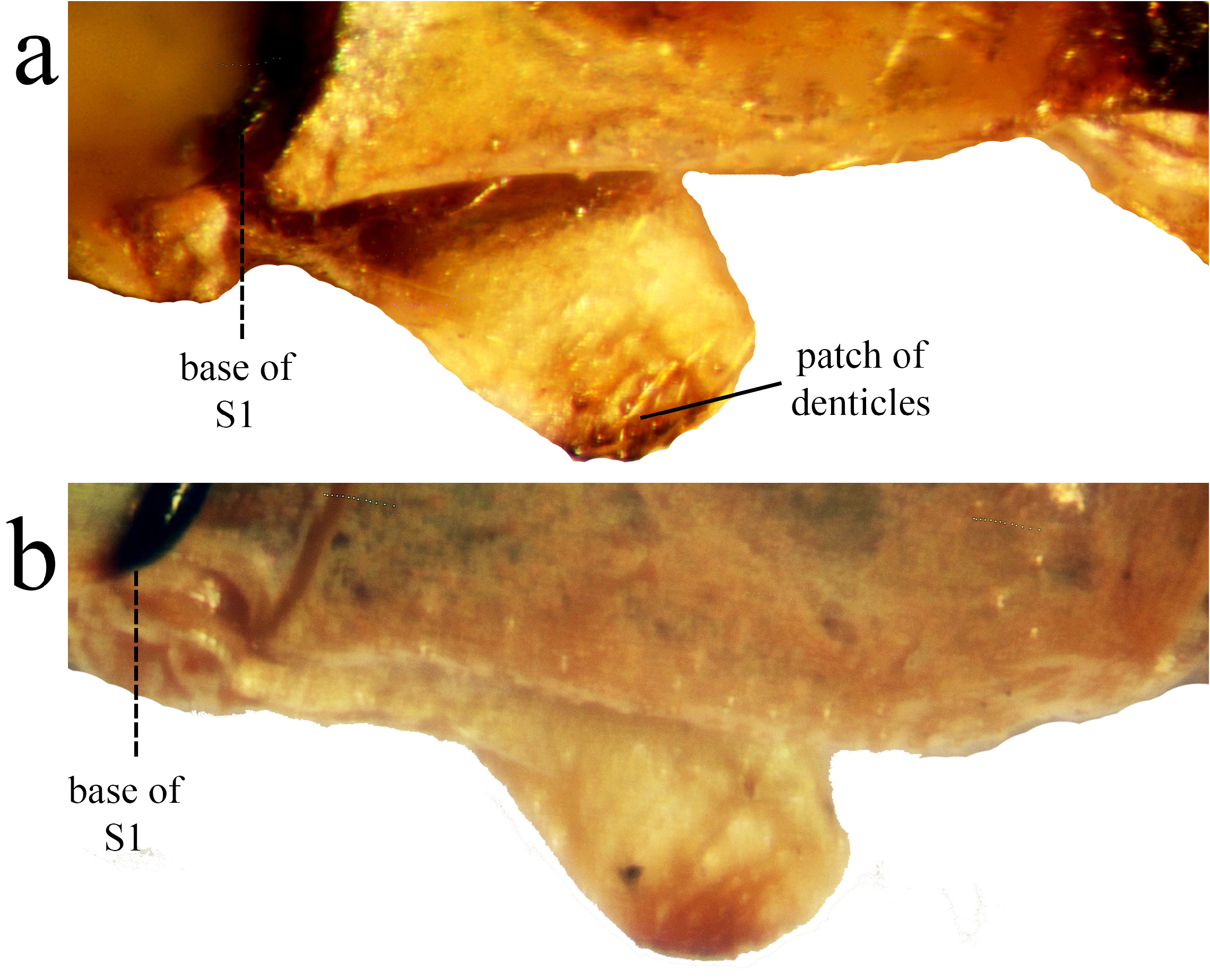
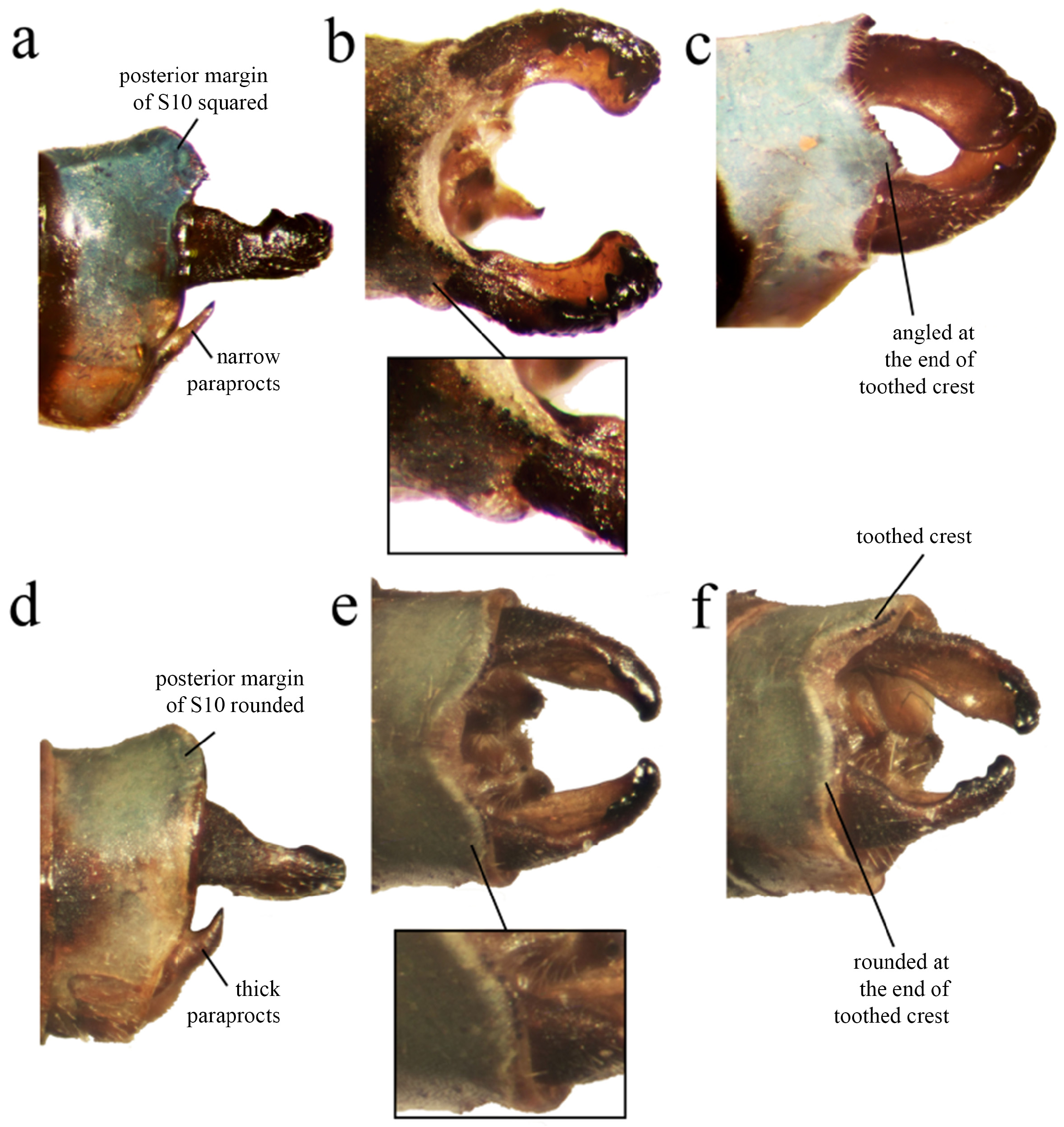
Abdomen ( Figs. 1–2 View FIGURE 1 View FIGURE 2 ). S1–2 yellow/orange dorsally, duller colored towards the lateral and ventral portions; tubercle with digit-like shape with a darker and rounded apex bearing a patch of several apical setae; S3–6 yellow/ orange with a black apical ring; S7 yellow basally, darkening posteriorly; S8–9 black, with a large “T” shaped blue spot; S10 blue, the toothed crest forming a dorsal angled corner with the lateral margin ( Figs. 4a–c View FIGURE 4 ).
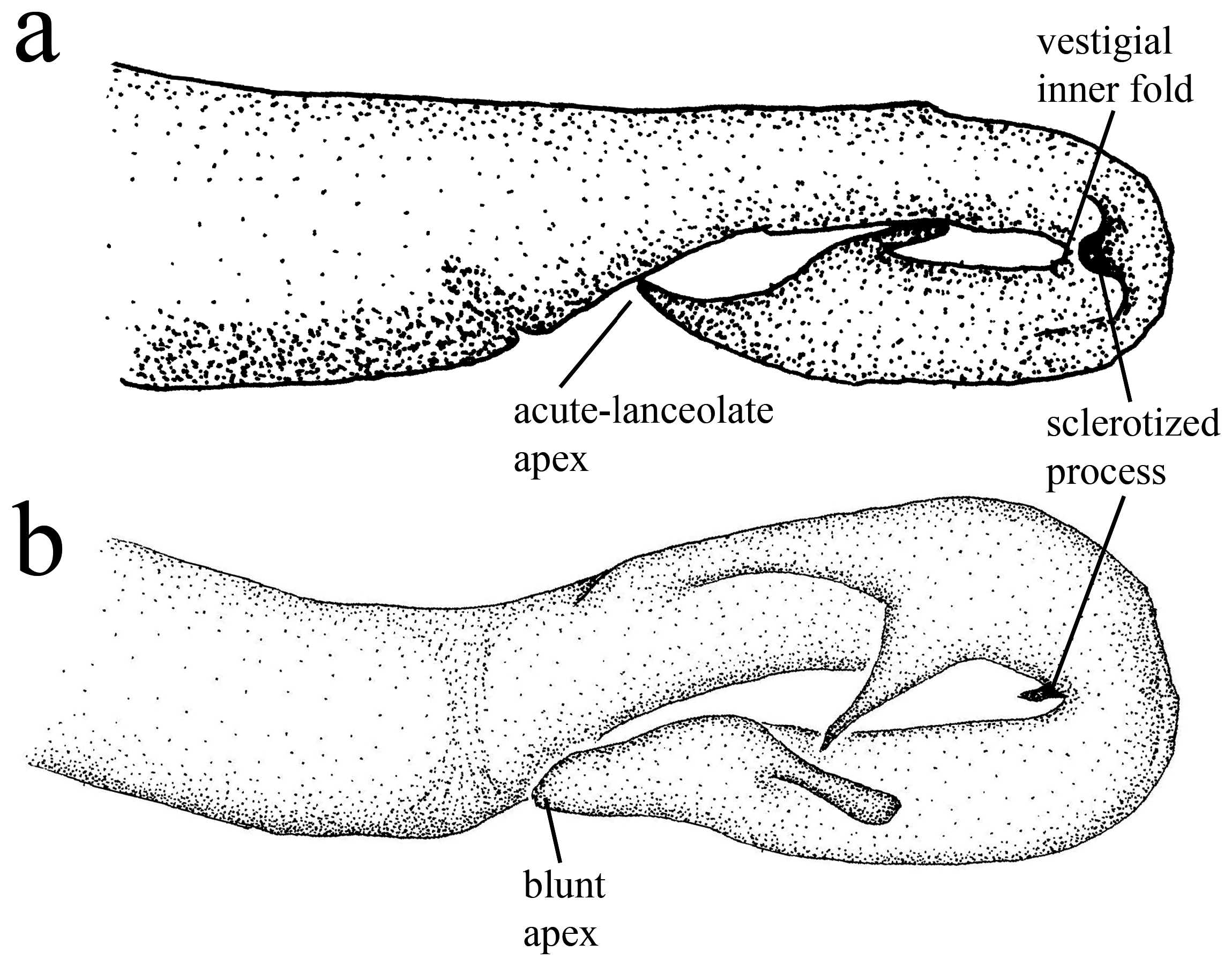
Genital ligula ( Fig. 3 View FIGURE 3 ). Segment one with a pair of digit-like lateral sclerotized processes, similar to lobes, in the flexure; inner fold vestigial. Segment two with a lanceolate apex, and two small lateral lobes.
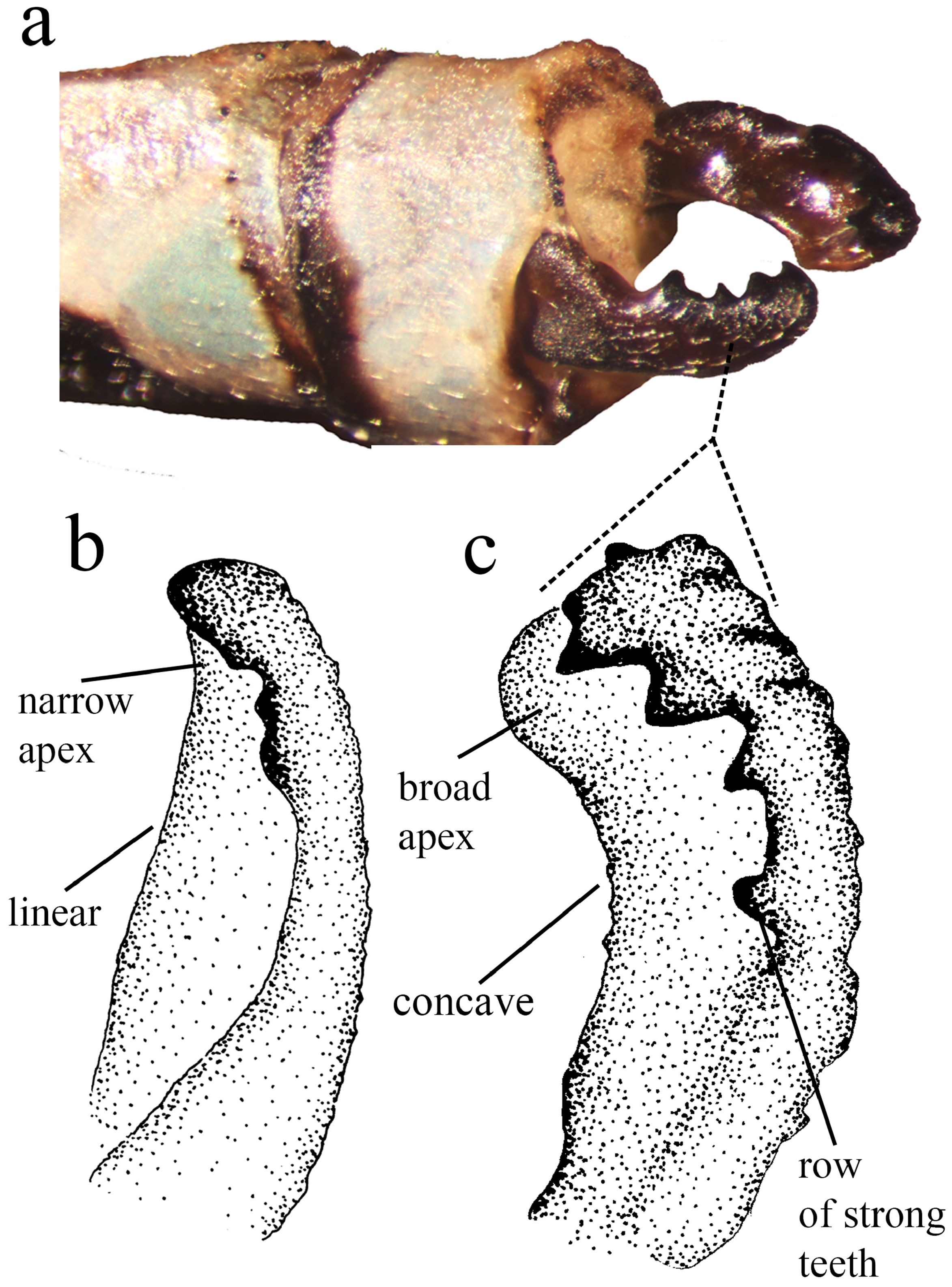
Anal appendages ( Figs. 4–5 View FIGURE 4 View FIGURE 5 ). Cercus forcipate, slightly longer than S10; dorsal plate bearing a row of four strong teeth, plus one smaller apical tooth; reaching the midlength of the basal plate; basal plate flat, broadening on the apical 1/3, ending on a rounded tip. Paraproct narrow, apex acute and directed upwards.
Measurements. Abdomen 23.8; FW 14.6; HW 13.7.
Variation in paratypes. Overall coloration and morphology agree with the holotype. Measurements varied as follows: Total 30–31; abdomen 24–25; FW 14–14.5; HW 13–13.5.
Diagnosis. Minagrion veredae sp. nov. is closer to M. waltheri ( Selys, 1876) and M. mecistogastrum (Selys, 1876) in terms of body morphology and coloration. It can be easily differentiated from M. mecistogastrum by body size (the largest species of the genus, with an average body size of 57mm), genital ligula and morphology of anal appendages. The following characters allow for the differentiation between M. veredae and M. waltheri (the latter in parenthesis): cercus ( Figs. 4b View FIGURE 4 , 5c View FIGURE 5 ) markedly forcipate, with the ventral margin deeply concave (less forcipate, with ventral margin mostly linear; Figs. 4e View FIGURE 4 , 5b View FIGURE 5 ); cercus dorsal plate bearing a row of four large strong teeth ( Figs. 5a, c View FIGURE 5 ), plus one smaller apical tooth (cercus dorsal plate bearing a row of short teeth, lacking apical tooth; Fig. 5b View FIGURE 5 ); cercus basal plate flat ( Fig. 5c View FIGURE 5 ), broadening on the apical 1/3, ending on a rounded tip (cercus basal plate flat, narrowing at the apical 1/3, ending on a blunt tip; Fig. 5b View FIGURE 5 ); paraprocts ( Fig. 4a View FIGURE 4 ) narrow and acute (paraprocts thicker and also acute; Fig. 4d View FIGURE 4 ); S10 toothed crest ( Figs. 4a–c View FIGURE 4 ) forming a dorsal angled corner with the lateral margin (S10 toothed crest not forming an angled corner, instead a rounded border; Figs. 4e–f View FIGURE 4 ); genital ligula ( Fig. 3a View FIGURE 3 ) with a pair of digit-like lateral sclerotized processes (sclerotized processes small, not digit-like; Fig. 3b View FIGURE 3 ); segment two of ligula ( Fig. 3a View FIGURE 3 ) with a lanceolate apex, and two small lateral lobes (segment two of ligula with a blunt apex, and two longer lateral lobes; Fig. 3b View FIGURE 3 ).
Habitat and distribution. The habitat of M. veredae is similar to what is reported for other species of the genus, the palm swamp environments ( Santos 1965; Machado & Bedê 2016; Vilela et al. 2020; Fig. 6 View FIGURE 6 ). These environments are composed of permanent wet soils, which provide suitable reproductive sites for these species throughout the year (Vilela et al. 2016). Nonetheless, the larval stages of Minagrion are still unknown, pending on further searches on veredas to fill this taxonomic gap.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |