Macromalthinus schmidli Constantin, 2010
|
publication ID |
https://doi.org/ 10.11606/1807-0205/2018.58.58 |
|
publication LSID |
lsid:zoobank.org:pub:97C262C2-6F02-4916-9533-43653FE5561F |
|
persistent identifier |
https://treatment.plazi.org/id/03821672-FFCF-1D50-5354-FE71FC0FFAEC |
|
treatment provided by |
Valdenar |
|
scientific name |
Macromalthinus schmidli Constantin, 2010 |
| status |
|
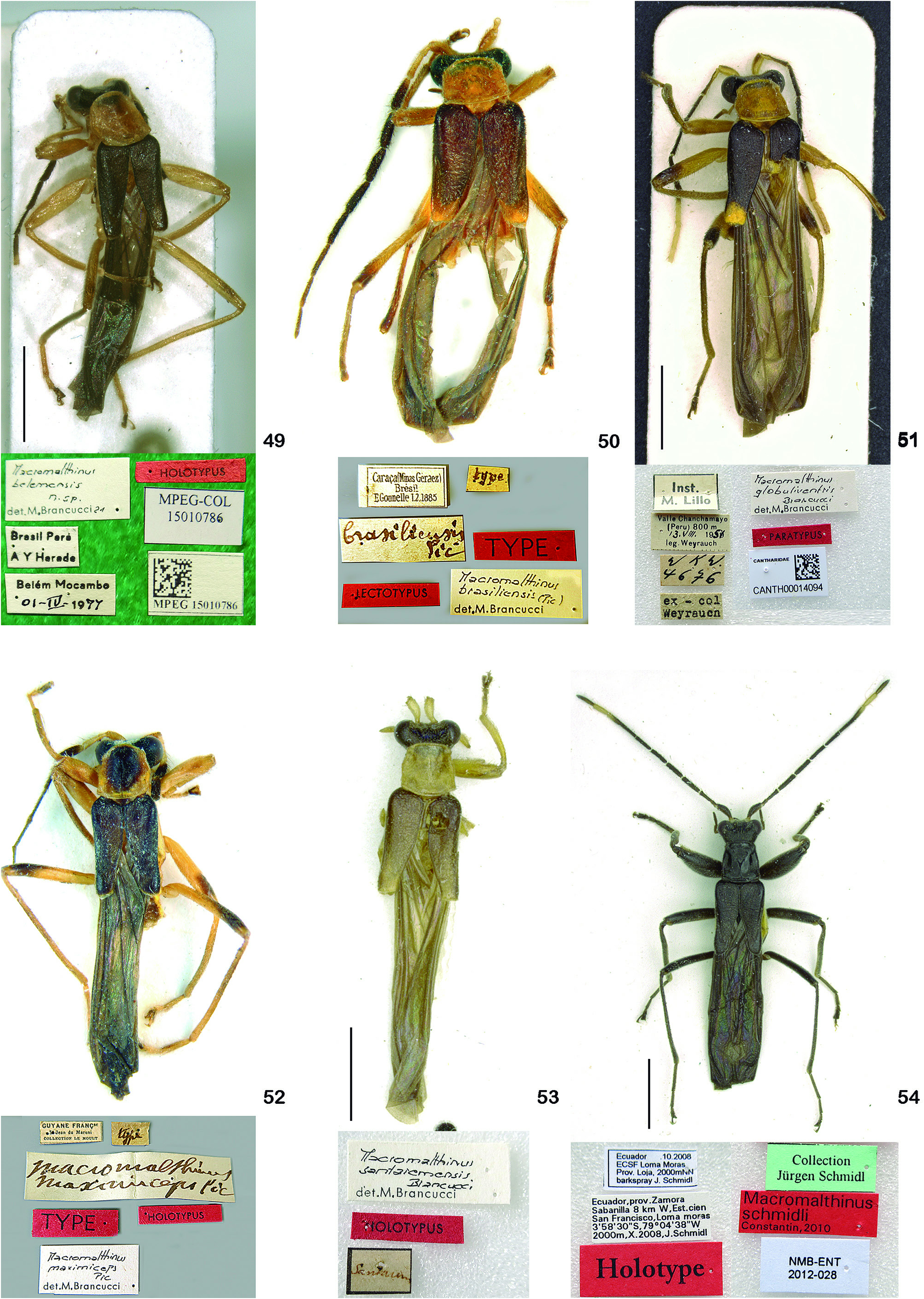
Macromalthinus schmidli Constantin, 2010 ( Fig. 54 View Figures 49-54 )
Macromalthinus schmidli Constantin, 2010a: 24 .
Material examined: HOLOTYPE ♂: ECUADOR. Zamora , EstaciónCientíficaSanFrancisco, LomaMoras, 03°58’30”S, 79°04’38”W, 2,000 m, X.2008, J. Schmidl ( NHMB) ( Fig. 54 View Figures 49-54 ). GoogleMaps
Differential diagnosis: Head, pronotum and elytra entirely black; males: fore femur strongly swollen with a small, sharp, apical ventral tooth; aedeagus with right prolongation of tegmen broad; left setiferous prolongation short; females: ventrite VII wide, lateral margins broadly arched, posterior margin slightly projected.
Macromalthinus schmidli differs from all the other species by head, pronotum and elytra entirely black, and fore femur of males strongly swollen, with a small apical ventral tooth.
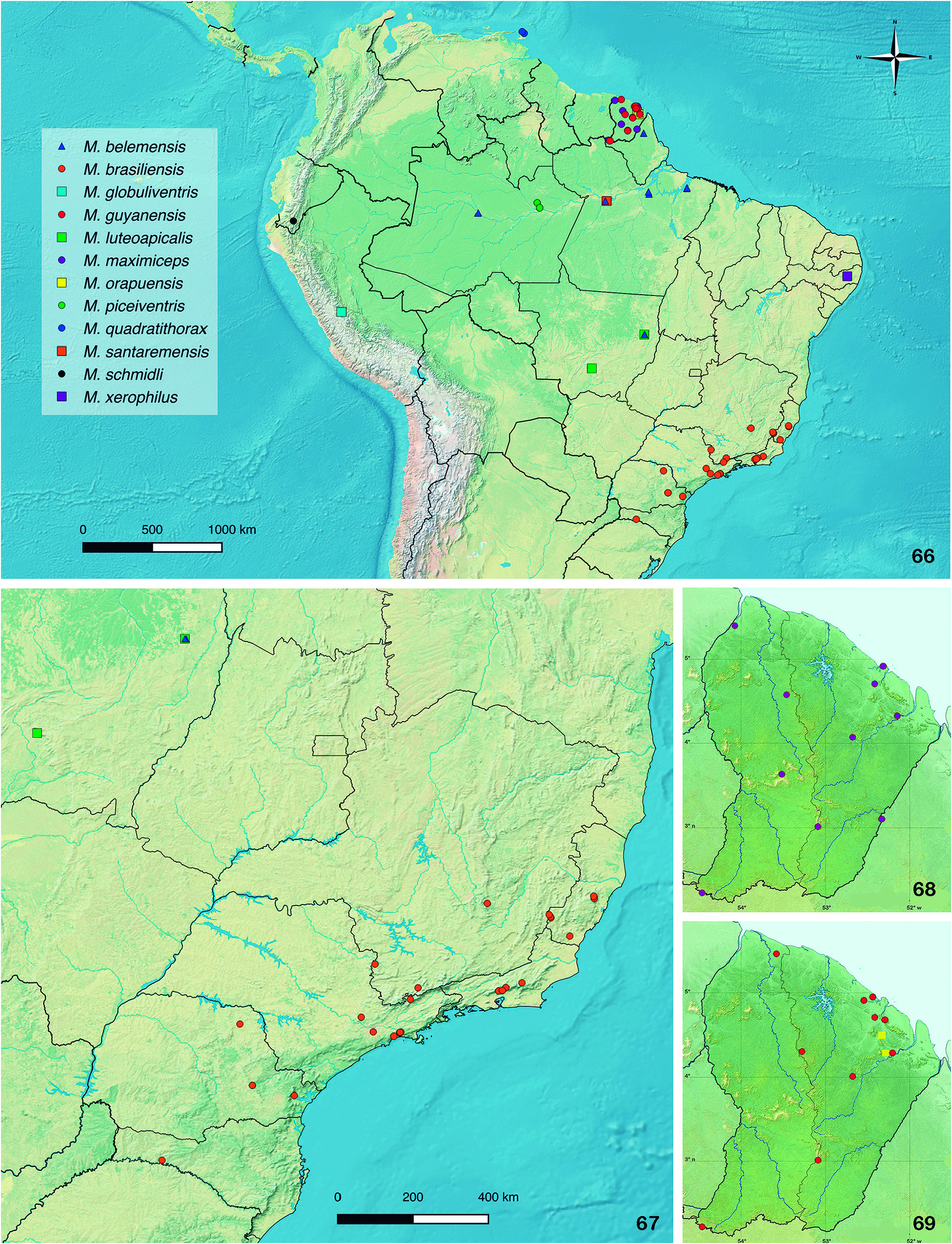
Distribution: Ecuador ( Fig. 66 View Figures 66-69 ).
Remarks on morphology
One of the diagnostic characters ascribed for Macromalthinus is the abdomen of males curved under itself in rest position ( Brancucci, 1981a) ( Fig. 59 View Figures 59-65 ). Actually, this curvature is the consequence of dehydration of the broad membranes between abdominal ventrites ( Fig.60 View Figures 59-65 ) and depends on the killing and preserving methods applied to the specimens. When alive, the abdomens of males are normally straight ( Fig. 61 View Figures 59-65 ).
Female genitalia of Macromalthinus species ( Fig. 62 View Figures 59-65 ) have characters in common with both members of Chauliognathini and Ichthyurini genera, according to the general morphological configuration outlined by Brancucci (1980). In all studied species, the vagina is sacciform, abruptly constricted before bursa copulatrix; oviduct joining vagina ventrally; accessory gland dorsally; bursa copulatrix globose, very small; spermatheca consisting of a tuft of spiralling filaments joining the bursa copulatrix anteriorly by a short duct.
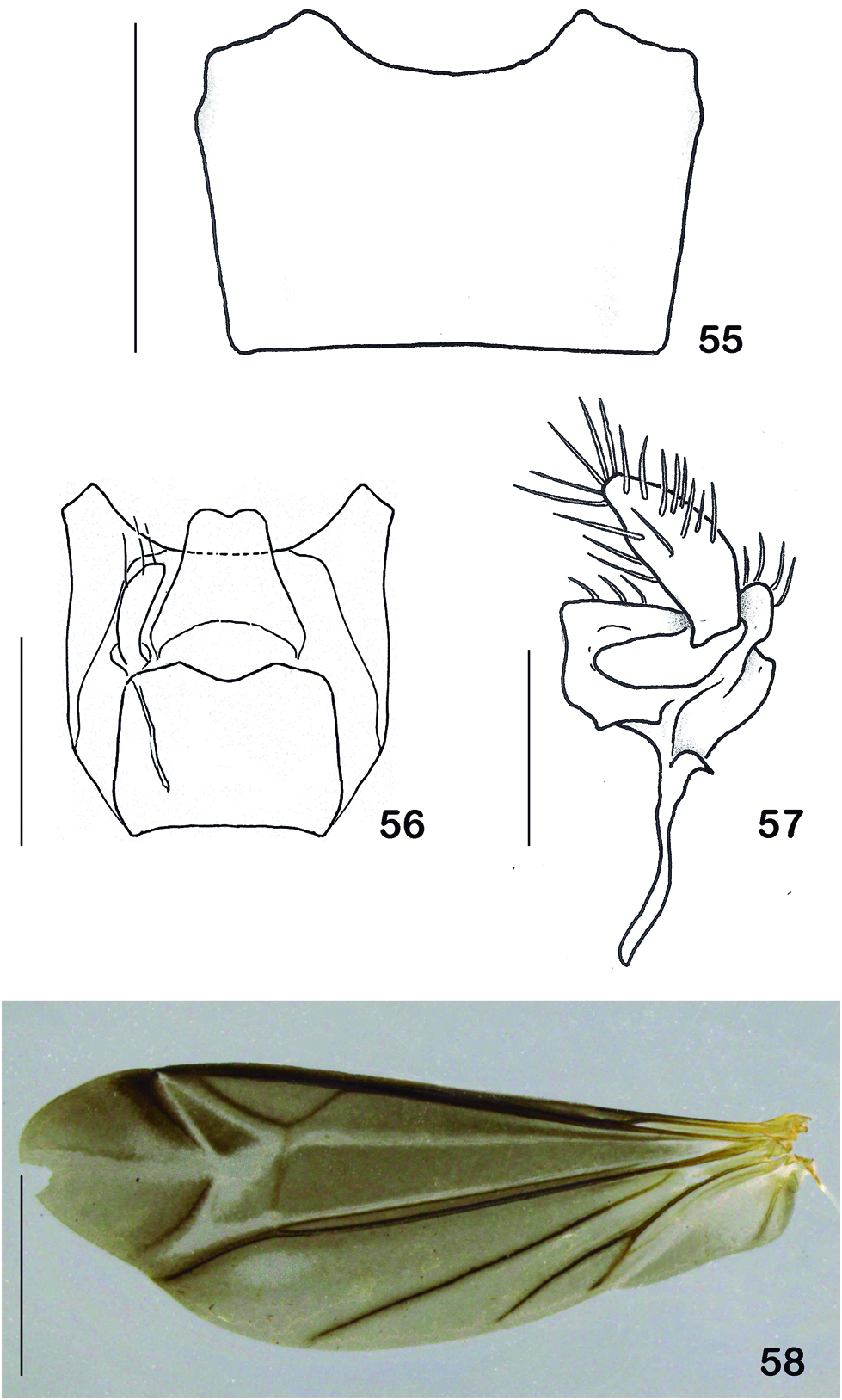
According to Brancucci (1980), the wings of Chauliognathinae are somewhat constant, with minor differences distinguishing the two tribes,Chauliognathini and Ichthyurini. Macromalthinus species show the same general aspect of that described by Brancucci (1980) as ‘ type Chauliognathus ’: wing veins reduced; veins r and r-m present; radial cell 2R₁ closed; vein Rr extending just until meeting point with r-m; vein Mr sclerotized; transversal vein cu-a absent; margin of anal area slightly sinuous. However, some of the differences observed in Macromalthinus species from that basic model resemble the Ichthyurini wings highlighted by Brancucci (1980): wings much longer and narrower; base much narrower than distal half; vein Cu straight, without distinct limits from M₃ ₊ ₄; vein Ax2 fading, not visible throughout its length; vein Mr running very close to M₃ ₊ ₄ ( Fig. 58 View Figures 55-58 ).
Morphologically, two main groups of species can be recognized in Macromalthinus . In the first, composed of M. maximiceps , M. belemensis , M. santaremensis , M. globuliventris , M. schmidli , M. guyanensis sp. nov., M. orapuensis sp. nov., M. piceiventris sp. nov. and M. luteoapicalis sp.nov., the specimens are larger,more robust and with a strong constriction near the anterior angles of pronotum ( Figs. 7-10 View Figures 7-12 ), and aedeagus with a large and broad right prolongation of tegmen ( Figs. 31-34 View Figures 31-36 ). The second group is composed of M. brasiliensis , M. xerophilus sp. nov. and M. quadratithorax sp.nov. Specimens of these species are smaller,with a subquadrate pronotum ( Figs. 11-12 View Figures 7-12 ), without strong constrictions near anterior angle; right prolongation of tegmen shorter and narrower ( Figs. 35-36 View Figures 31-36 ).
The setiferous prolongation can be very long ( M.maximiceps , M. belemensis , M. santaremensis , M. luteoapicalis sp.nov.)(e.g., Fig.32 View Figures 31-36 ) to short and broad ( M.globuliventris , M. schmidli , M.piceiventris sp. nov., M. guyanensis sp. nov., M. orapuensis sp. nov., M. quadratithorax sp. nov.) (e.g., Figs. 31, 33-35 View Figures 31-36 ), or totally absent ( M. xerophilus sp. nov., M. brasiliensis ) ( Figs. 36 View Figures 31-36 , 65 View Figures 59-65 ). According to Brancucci (1981a), the genus encompasses typical, morphologically resembling species, except for M. brasiliensis , which seemed to be the transition between Macromalthinus and Maronius . Macromalthinus xerophilus sp. nov. and M. quadratithorax sp. nov. resemble M. brasiliensis in the pronotum with feebly constricted lateral margins, and tegmen with narrower right prolongation and absence of setiferous prolongation.
Distribution and natural history of Macromalthinus
Distributional data known prior to this work showed restricted distribution records and wide disjunction of species ( Brancucci, 1981a; Constantin, 2010a, 2016). The species of this genus were recorded only from French Guiana ( M. maximiceps ), Ecuador ( M. schmidli ), Peru ( M. globuliventris ), northern ( M. belemensis , M. santaremensis ) and southeastern Brazil ( M. brasiliensis ). New records fill wide distributional gaps of Macromalthinus species in South America and approximate formerly widely separated species ( Figs. 66-69 View Figures 66-69 ).
Some species are still known only from single or nearby localities, like M. globuliventris , M. schmidli and M. santaremensis , whereas for others the known distribution is broadly expanded, like for M. maximiceps , M. belemensis and M. brasiliensis . Members of the genus are record- ed for the first time in Trinidad and Tobago ( Trinidad Island) and in northeastern, middle-western and southern Brazil, embracing a wide range of biomes, vegetation types, climates and altitudinal ranges. Most of the species inhabit very humid, sometimes flooded regions and gallery forests in central Amazon (like M. belemensis , M. santaremensis and M. piceiventris sp. nov.) or along the northern and southern reaches of Amazon (like M. maximiceps , M. orapuensis sp. nov., M. guyanensis sp. nov. and M. luteoapicalis sp. nov.). Macromalthinus brasiliensis is widespread in several vegetation types along the southern and southeastern Brazilian Atlantic forest, like the coastal tropical moist broadleaf restinga forest at the seashore, campos rupestres highlands (shrubby montane savannas), montane forests, dryer inland areas and in small forest patches in metropolitan areas (São Paulo city). In contrast, M. xerophilus sp. nov. is only recorded from a transition zone between the Atlantic forest and the semiarid caatinga. The specimens are usually collect- ed with malaise traps or by sweeping the lower shrubby vegetation.
Nothing is known about feeding habits of Macromalthinus . It seems that there are not specific food sources,as seen by the wide distribution of some species throughout such distinct vegetation types, biomes and altitudinal ranges.
| NHMB |
Natural History Museum Bucharest |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Macromalthinus schmidli Constantin, 2010
| Biffi, Gabriel & Constantin, Robert 2018 |
Macromalthinus schmidli
| Constantin, R. 2010: 24 |