Dendropsophus haraldschultzi (Bokermann, 1962)
|
publication ID |
https://doi.org/10.11646/zootaxa.4780.3.11 |
|
DOI |
https://doi.org/10.5281/zenodo.4323886 |
|
persistent identifier |
https://treatment.plazi.org/id/0382DB4B-FF89-FF8A-C5E5-70A9AB14FC8E |
|
treatment provided by |
Felipe |
|
scientific name |
Dendropsophus haraldschultzi |
| status |
|
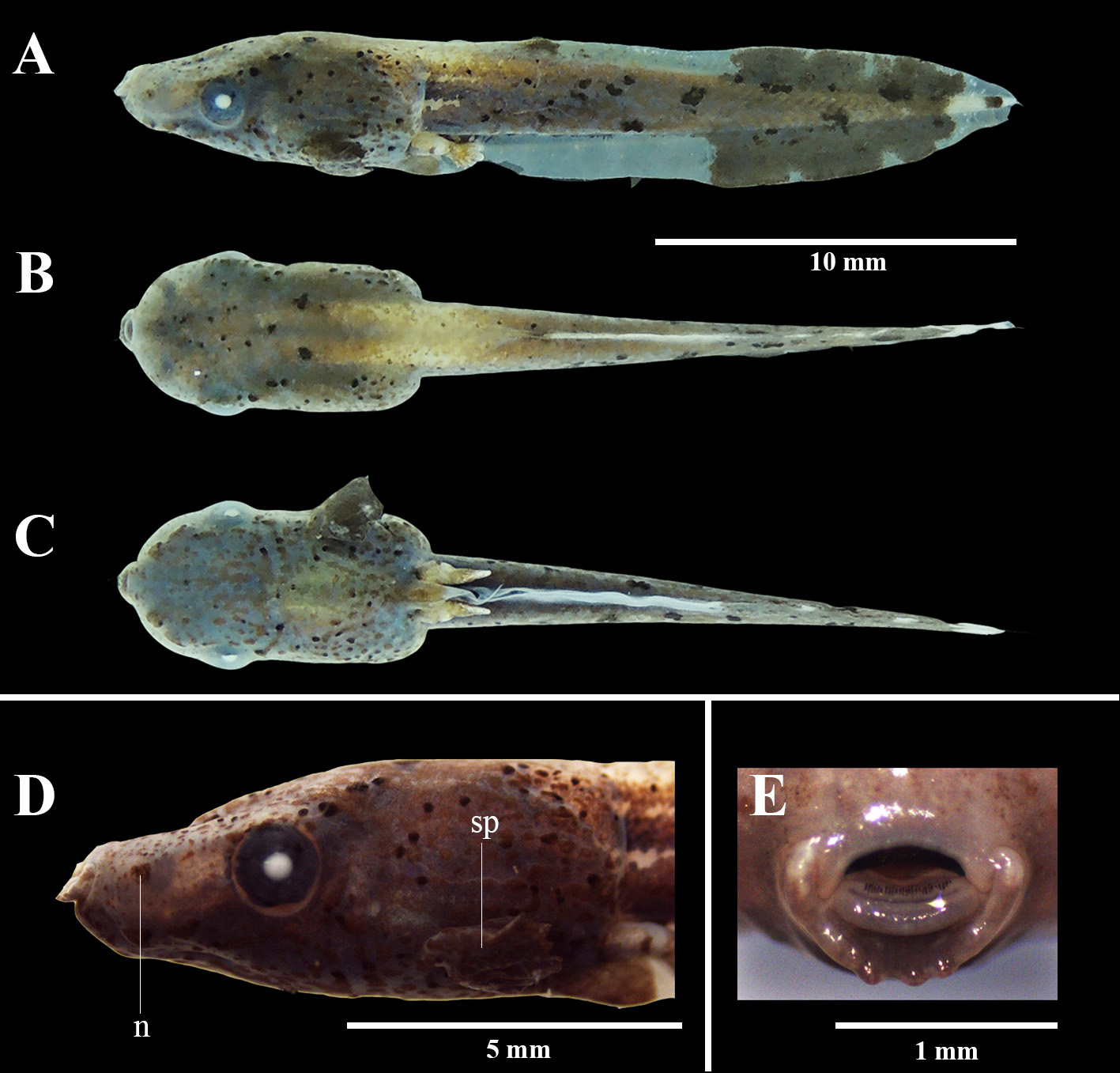
obtained one egg clutch from an amplectant pair kept overnight in a plastic bag containing water collected from their natural habitat. The frogs were found in a floodplain area of the municipality of Santana, Amapá State, Brazil ( 0.0363°N, 51.1625°W, Datum: WGS84; 26 m. a.s.l.), in May 2018. The clutch was maintained in a glass container ( 8 cm 3) in the laboratory and the tadpoles were fed with fish food. Twelve specimens were euthanized in solution of benzocaine diluted in water and fixed and preserved in a 1:1 solution containing ethanol 70% and formalin 15%. The tadpoles and adults were deposited in the Herpetological Collection of the Universidade Federal do Amapá, Macapá , Amapá State, Brazil (Tadpole lots CECCAMPOS 2655 to 2666; Adults CECCAMPOS 2386–2388, 0562, 0563, 0569–0571, 0573– 0575, 0577). GoogleMaps Additionally , this study also included six tadpoles collected on 08–09 February and 08 April 2013 in floating meadows in the Catalão Lake ( 3.1667°S, 59.9142°W, Datum: WGS84), in the municipality of Iranduba , Amazonas State , Brazil, and deposited in the Coleção Zoológica Paulo Bührnheim of the Universidade Federal do Amazonas , Manaus , Amazonas State , Brazil (CZPB-LA tadpole lots 257/576, 258/580, 282/633, 282/634, 283/636, 285/648). Measurements were obtained with use of an ocular micrometer (nearest 0.01 mm) in a stereoscopic microscope. Descriptive terminology and morphometric measurements follow Altig & McDiarmid (1999): total length (TL), body length (BL), body width (BW), body height (BH), tail length (TAL), maximum tail height (MTH), tail muscle height (TMH), tail muscle width (TMW), internarial distance (IND), interorbital distance (IOD), eye diameter (ED), eye-nostril distance (END), nostrilsnout distance (NSD), and oral disc width (ODW). We also measured the spiracle length (SL) and the vent-tube length (VTL). Developmental stages follow Gosner (1960) GoogleMaps .
Tadpole description. The description is based on two tadpoles at Stage 35 (CZPB-LA 258/580, 285/648). Body depressed in lateral view ( Fig. 1A View FIGURE 1 ) and violin-shaped with a slight constriction posterior to the orbits in dorsal and ventral views ( Fig. 1B and C View FIGURE 1 ); body length 31.8–33.3% of the total length. Maximum body height in the middle of the body representing 42.0–46.9% of body length and 84.0–88.4% of body width. Body width at spiracle level near to body width at eye level. Snout rounded in dorsal and lateral views. Eyes laterally positioned and directed; eye diameter representing 14.6–16.2% of body length. Nostrils oval and small, without a fleshy ring in its inner margins, anterolaterally positioned and closer to the snout than to eyes, opening anterolaterally directed ( Fig. 1D View FIGURE 1 ). Internarial distance representing 69.7– 75.0% of body width. Spiracle single, sinistral, long, and wide, opening in the posterior third of the body, located lateroventrally and posteriorly directed. Spiracle centripetal wall fused to the body wall; external wall longer than centripetal wall and with irregular margin ( Fig. 1D View FIGURE 1 ). Vent tube single, short, dextral, fused to the ventral fin. Tail length representing 66.7–68.1% of total length. Caudal musculature gradually tapering to a pointed tip in lateral view. Tail tip pointed. Tail muscle height representing 67.5% of maximum tail height. Tail muscle width representing 47.5–48.1% of body width. Dorsal fin shallow, originating on the anterior third of the tail. Ventral fin convex originating at the posterior extremity of the ventral terminus and with linear margins following the tail axis. Lateral line system evident, with stitches of lateral lines rounded; presence of oral, supraorbital, infraorbital, preorbital, angular, medial, dorsal, and ventral lines. Oral disc ( Fig. 1E View FIGURE 1 ) terminal, not emarginated, with wide gap on the anterior labium and rounded uniseriate marginal papillae located in the ventral margin of the posterior labium. Lateral marginal papillae partially fused forming a dermal vertical fold on both sides of oral disc. Submarginal papillae absent. Oral disc width representing 27.6–28.0% of body width. Upper and lower jaw sheaths large; upper jaw sheath arch-shaped and lower jaw sheath U-shaped; upper jaw sheath thinner than the lower jaw sheath; both jaw sheaths black, keratinized, and finely serrated. Labial tooth row formula (LTRF) 0/1; P1 medial and shorter than jaw sheaths. Posterior labium with a dermal ridge (likely a second labial ridge) between tooth row and papillae.
Coloration. In life (Stage 25), dorsum, lateral and ventral body grayish-brown with small spots on the anterior portion of the body and yellowish pigment spots from the posterior portion of the body to the tail. Digestive tract barely visible ventrally. Tail musculature color similar to the body with small dark spots on lateral view. Fins translucent with irregular marks grayish-brown on the posterior portion. Iris black. In preservative (Stage 35), body and caudal musculature dark brown with small rounded spots on the dorsum, lateral of the body and caudal musculature. Fins translucent with irregular dark brown marks on the posterior third. Iris dark brown. Glandular patches present on the dorsal and lateral surface of the body.
Variation. Variation in 16 morphometric characteristics of tadpoles from Gosner Stages 25, 31, 34, 35 and 36 is given in Table 1. There is no variation in LTRF along all development stages analyzed, but there is variation in the P1 row size, usually short at Stage 25 and longer at older stages. Spiracle is more evident in the older stages analyzed and its size is maximum at Stage 35 ( 2.05–2.25 mm). Dorsal fin originating at body-tail junction at stage 25 and on the anterior third of the tail at older stages.
Natural history. The couple of Dendropsophus haraldschultzi in amplexus was found in a floodplain, perched on aquatic macrophytes ( Montrichardia sp.) 69 cm from the water surface. Snout-vent length and body mass were 19.72 mm and 0.4 g for the male, and 22.62 mm and 0.6 g for the female. The amplexus is axillary. Clutch size was 70 eggs (one clutch); eggs were white and clumped in a gelatinous mass in the water surface. We observed males of D. haraldschultzi , D. leucophyllatus and Scarthyla goinorum (Bokermann) in calling activity at night (between 18:30 to 20:00 h) in the same site where the couple was found. The advertisement call of D. haraldschultzi (not recorded) is composed of two types of pulsed notes with similar dominant frequencies emitted separately (only one individual was observed).
The tadpoles of Dendropsophus haraldschultzi at stages 31 and 34 to 36 are morphologically similar to what was illustrated by Lynch & Suárez-Mayorga (2011) as the tadpole of D. haraldschultzi from Colombia. Forty-six Dendropsophus tadpole species from six species groups were described and/or figured previously ( Bokermann 1963; Duellman & Fouquette 1968; Kenny 1969; Duellman 1970, 1972, 1978, 2005; Duellman & Crump 1974; Weygoldt & Peixoto 1987; Hero 1990; Heyer et al. 1990; Lavilla 1990; Cruz & Dias 1991; Gomes & Peixoto 1991a, 1991b; Wild 1992; Santos et al. 1998; Peixoto & Gomes 1999; Cruz et al. 2000; Wogel et al. 2000; Pugliese et al. 2000, 2001; Carvalho-e-Silva et al. 2003; Lynch 2006; Rossa-Feres & Nomura 2006; Lynch & Suárez-Mayorga 2011; Lourenço-de-Moraes et al. 2012; Abreu et al. 2014, 2015; Fouquet et al. 2015; Schulze et al. 2015; Ruas et al. 2018). Tadpoles of D. haraldschultzi can be easily differentiated from all of them by its long, wide, and lateroventral spiracle (spiracle is sinistral and short in the remaining species). An elongate spiracle was reported in tadpoles of other hylids, including species of Boana albopunctata Spix group ( Wild 1992; Rossa-Feres & Nomura 2006) and Sphaenorhynchus Tschudi (e.g., Nunes et al. 2007; Araujo- Vieira et al. 2015), and in the microhylid genus Otophryne Boulenger (e.g., Pyburn 1980). Like in Sphaenorhynchus species, tadpoles of D. haraldschultzi are found in the root of aquatic macrophytes in floating meadows (Bönning et al. 2017), but functional aspects of the long spiracle are unknown ( Araujo-Vieira et al. 2015).
In most general features, e.g., small size, snout rounded, oral disc anterior with mouthparts usually reduced, Dendropsophus haraldschultzi larvae are similar to other tadpoles of the genus. In some individual traits however these tadpoles resemble larvae of different species groups. For instance, the post-ocular constriction that gives the body a violin-shape occurs also in most tadpoles of the D. leucophyllatus group [ D. arndti Caminer, Milá, Jansen, Fouquet, Venegas, Chávez, Lougheed, and Ron , D. bifurcus (Anderson) , D. elegans (Wied-Neuwied) , D. leucophyllatus , D. reticulatus (Jiménez de la Espada) , D. salli Jungfer, Reichle, and Piskurek , D. sarayacuensis (Shreve) , and D. triangulum (Günther) ], species of the D. microcephalus group [i.e., D. branneri (Cochran) , D. microcephalus , D. nanus (Boulenger) , D. rubicundulus (Reinhardt and Lütken) , D. sanborni (Schmidt) , D. studerae (Carvalho-e-Silva, Carvalho-e-Silva and Izecksohn)], within the D. marmoratus group [i.e., D. melanargyreus (Cope) ], the D. parviceps group [i.e., D. timbeba (Martins and Cardoso) ], and in D. minutus . The tail tip without a flagellum is also characteristic of D. decipiens (Lutz) , D. haddadi , D. oliveirai (Bokermann) , and D. riveroi (Cochran and Goin) of the D. microcephalus group, and D. microps (Peters) and D. ruschii (Weygoldt and Peixoto) of the D. parviceps group.
The configuration of the oral disc is widely variable in Dendropsophus , and varied extents of oral structure reductions are reported in several groups (e.g., Duellman 1978, 2005; Schulze et al. 2015). On one hand, marginal papillae reduced to lateral commissures on upper lateral portion and lower labium appear in D. haraldschultzi and most species of the D. leucophyllatus group ( D. arndti , D. ebraccatus (Cope) , D. elegans , D. rossalleni (Goin) , D. sarayacuensis , D. salli ), and D. parviceps group [ D. giesleri (Mertens) , D. microps ]. The fusion of lateral marginal papillae forming a dermal vertical fold on both sides of oral disc has been described only in D. counani Fouquet, Orrico, Ernst, Blanc, Martinez, Vacher, Rodrigues, Ouboter, Jairam, and Ron. On the other hand, the number of tooth rows in the genus varies from 0/0 in many species of the D. leucophyllatus , D. marmoratus , D. minutus , D. parviceps and D. microcephalus species groups to 2/ 2 in D. anceps (Lutz) . Besides in D. haraldschultzi , a LTRF 0/1 has been reported in D. elegans , D. rossalleni , D. salli ( D. leucophyllatus group), D. giesleri , D. microps , and D. ruschii of the D. parviceps group; in D. melanargyreus , D. nahdereri (Lutz and Bokermann) , D. novaisi (Bokermann) , D. seniculus (Cope) , and D. soaresi (Caramaschi and Jim) of the D. marmoratus group, and in D. minutus . In addition, further labial ridges without labial teeth are found also in some species of the D. microcephalus group (one ridge in D haddadi , two ridges in D. berthalutzae and D. decipiens ). This combination of characters indicates that larval morphology is ambiguous to assign D. haraldschultzi to any species group.
As a final comment, clutch size of Dendropsophus haraldschultzi found in our study is smaller than those reported in the literature for gravid females with mature ovarian eggs ( 216 eggs; Hödl 1990). Most species of Dendropsophus occurring in Amazonia lay their eggs on the water, on the surface of leaves of emergent vegetation [ D. bifurcus , D. bokermanni (Goin) , D. brevifrons (Duellman and Crump) , D. reticulatus , D. sarayacuensis , D. timbeba and D. reticulatus ]. Conversely, D. haraldschultzi is similar to D. koechlini (Duellman and Trueb) , D. leali (Bokermann) , D. marmoratus , D. minutus , and D. parviceps in that they deposit the eggs directly in the water ( Duellman 1978, 2005; Rodríguez & Duellman 1994).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.