Aconitum
|
publication ID |
https://doi.org/ 10.1016/j.phytochem.2018.06.011 |
|
DOI |
https://doi.org/10.5281/zenodo.10514082 |
|
persistent identifier |
https://treatment.plazi.org/id/038387BC-FFE7-FF85-0A1B-FC1DFA55FC2C |
|
treatment provided by |
Felipe |
|
scientific name |
Aconitum |
| status |
|
2.4. Application to Aconitum View in CoL samples
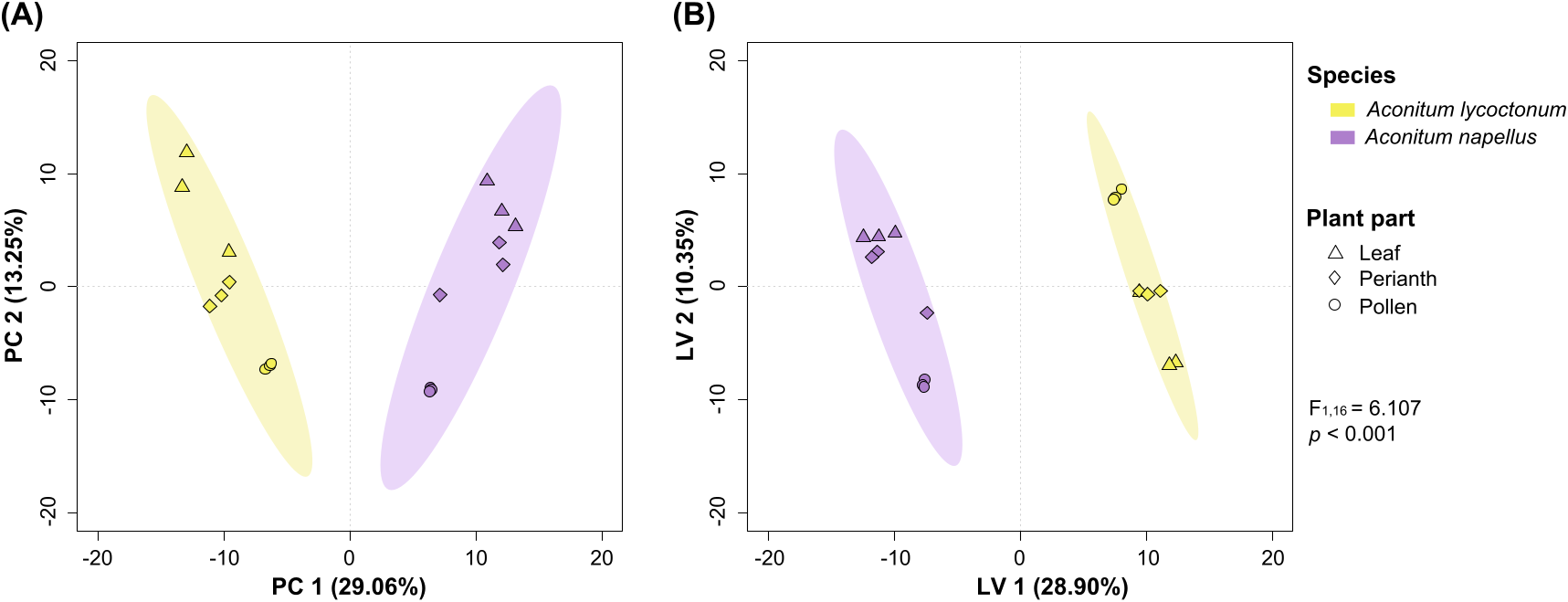
Using the MarkerLynx metabolomic approach, a total of 359 alkaloid candidates was detected in the leaf, perianth and pollen samples of A. lycoctonum and A. napellus . 296 molecular formulae matched with known alkaloids from the DNP database, including 282 already found in the Aconitum genus. The remaining 63 candidates (17.55%) represent potentially yet unknown alkaloids, thus this dataset may be useful for future phytochemical investigations of Aconitum tissues. To reduce data complexity, a principal component analysis (PCA) was performed on the refined dataset consisting of alkaloid candidates and revealed that the samples were clearly clustered in two different groups ( Fig. 3A View Fig ), with the two species being distributed on both sides of the PC1 axis that explained 29.06% of the total variance. This species-clustering was corroborated by a perMANOVA that detected a significant difference between the two Aconitum species (F 1,16 = 6.107, p <0.001). Such chemical variation between A. lycoctonum and A. napellus supports the use of specialized metabolites, especially diterpenoid alkaloids, as chemical markers valuable to plant taxonomy. The separation between the two Aconitum species was further investigated by partial least squares discriminant analysis (PLS-DA) ( Fig. 3B View Fig ), a supervised method that explains maximum separation among defined classes of samples. Once again, the ordination map showed a clear discrimination between the two species according to the LV 1 axis that explained 28.9% of the total variance ( Table 2 View Table 2 ). Following PLS-DA, the variable influence in projection (VIP) scores were used to select the most influential alkaloid-like metabolites that were mainly responsible for species separation (i.e. VIP ≥1). The 5 most influential candidates for each species were tentatively identified as known alkaloids by matching the obtained molecular formulae with the DNP database ( Table 3 View Table 3 ). All of them but one have been reported in the Aconitum and/or Delphinium genera ( Ranunculaceae family). Regarding the marker candidates of A. lycoctonum : (i) C 36 H 48 N 2 O 10 was tentatively assigned as lycaconitine, (ii) C 37 H 48 N 2 O 11 as potanidine B, (iii) C 24 H 33 NO 5 as a hetisane-type diterpenoid alkaloid, probably ternatine or cardionine, and (iv) C 25 H 35 NO 5 as a yuzurimine-type alkaloid, probably yuzurimine A or E. Four putative markers were tentatively identified in A. napellus , namely anthriscifoldine A (C 25 H 37 NO 7), 14- O -acetylegenicunine B (C 25 H 37 NO 7), bullatine C (C 26 H 41 NO 7) and napellonine (C 22 H 31 NO 3). It was not possible to propose unequivocal assignments for C 24 H 33 NO 4 (alkaloid-1AL) and C 24 H 37 NO 6 (alkaloid-1AN) because of the relatively high number of isomers reported in Aconitum for these molecular formulae.
From the review of the literature, lycaconitine, a C 19 diterpenoid alkaloid, is known to occur in A. lycoctonum since its isolation from the roots of the plant in 1884 ( Dragendorff and Spolm, 1884). Lycaconitine has been recently found in corollas of A. lycoctonum ( Barlow et al., 2017) . Potanidine B is a lycoctonine-type C 19 diterpenoid alkaloid that was isolated from the root of Delphinium potaninii Huth ( Ranunculaceae ) for its structural characterization ( Pu and Wang, 1994). Its occurrence in other tissues than roots is, to our knowledge, unreported. The hetisane-type diterpenoid alkaloid detected in A. lycoctonum might correspond to ternatine, previously isolated from the aerial parts of Delphinium ternatum Huth ( Ranunculaceae ) ( Narzullaev et al., 1997), or to cardionine previously isolated from above-ground parts of Delphinium cardiopetalum DC ( Ranunculaceae ) ( De la Fuente et al., 1990), both being C 20 diterpenoid alkaloids. The yuzurimine-type alkaloid might correspond to either yuzurimine A, a minor alkaloid from the bark and the leaves of Daphniphyllum macropodum Miq. ( Daphniphyllaceae ) ( Sakurai et al., 1967), or yuzurimine E, an alkaloid from the seed of Daphniphyllum calycinum Benth. ( Daphniphyllaceae ) ( El Bitar et al., 2004) and the leaves of Daphniphyllum glaucescens Blume ( Daphniphyllaceae ) ( Takatsu et al., 2004). With regards to the markers of Aconitum napellus , anthriscifoldine A is a C 19 diterpenoid alkaloid that was obtained from the whole herbs of Delphinium anthriscifolium var. savatieri Hance (Ranunulaceae) for its structure elucidation ( Song et al., 2009). 14- O -acetylegenicunine B is a norditerpene alkaloid isolated from aerial parts of Aconitum variegatum L. ( Ranunculaceae ) ( Diaz et al., 2005) that is a closely related species of A. napellus ( Luo et al., 2005) . Bullatine C is a C 19 diterpenoid alkaloid found in the nectar and galeas of Aconitum napellus ( Barlow et al., 2017) , while napellonine (i.e. dehydronapelline) is known as a C 20 diterpenoid alkaloid occurring in herb and flowers of A. napellus ( Chen et al., 1999) . Regarding the chemotaxonomic significance of these features, previous studies suggested that C 18 and C 19 diterpenoid alkaloids display a taxonomic importance relative to the C 20 diterpenoid alkloids ( Wang and Chen, 2010). Whereas species that exhibit more C 20 diterpenoid alkaloids may be regarded as more primitive, species with a major chemical composition based on C 19 diterpenoid alkaloids may be more evolved ( Wang and Chen, 2010). Such occurrence of C 20 diterpenoid alkaloids in ancient species is supported by the fact that C 20 diterpenoid alkaloids display much simpler backbone structures and are considered as biogenetic precursors of C 18 and C 19 diterpenoid alkaloids ( Wang and Chen, 2010).
3. Conclusion
The presented approach constitutes an efficient and applicable tool for profiling alkaloids in plant samples. By carefully optimizing the various parameters that are essential to correct EC assignment and by automating the process, numerous alkaloid candidates can be identified within a very short time (less than 30 min for a batch of ca. 500 features). The most promising molecules may then be further confirmed by MS/MS acquisition and comparison with existing databases. Such efficient analytical workflow is the basis of the modern approach of chemotaxonomy and might prove highly useful in the search for known and previously undescribed alkaloids from medicinal plants as well as to identify reliable marker compounds during exposure to toxic species such as Aconitum spp.
4. Experimental
4.1. Plant material
Plants were sampled in August 2013 in Switzerland in two localities: Kandersteg (place called Gastereholz , GPS coordinates: 46.45912 N, 7.67072 E; elevation: 1369.5 m) for Aconitum lycoctonum L. ( Ranunculaceae ), GoogleMaps and Boltigen (place called Ramsere , GPS coordinates: 46.63934 N, 7.38029 E; elevation: 1338.3 m) for Aconitum napellus L. ( Ranunculaceae ). Leaves and perianths were sampled from single specimens (n = 3 per species). Pollen was collected on several flowers and pooled to obtain sufficient amounts for analyses. Plant samples were stored at −80 ̊C until extraction and chromatographic analysis. GoogleMaps
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |