Artemisia abrotanum, L.
|
publication ID |
https://doi.org/10.1016/j.phytochem.2019.05.010 |
|
DOI |
https://doi.org/10.5281/zenodo.10580466 |
|
persistent identifier |
https://treatment.plazi.org/id/0383DE11-E44A-FFEE-0F0B-4A5474E28066 |
|
treatment provided by |
Felipe (2024-01-11 00:32:49, last updated 2024-01-30 14:54:56) |
|
scientific name |
Artemisia abrotanum |
| status |
|
2.1. Sesquiterpenoid profile of A. abrotanum View in CoL
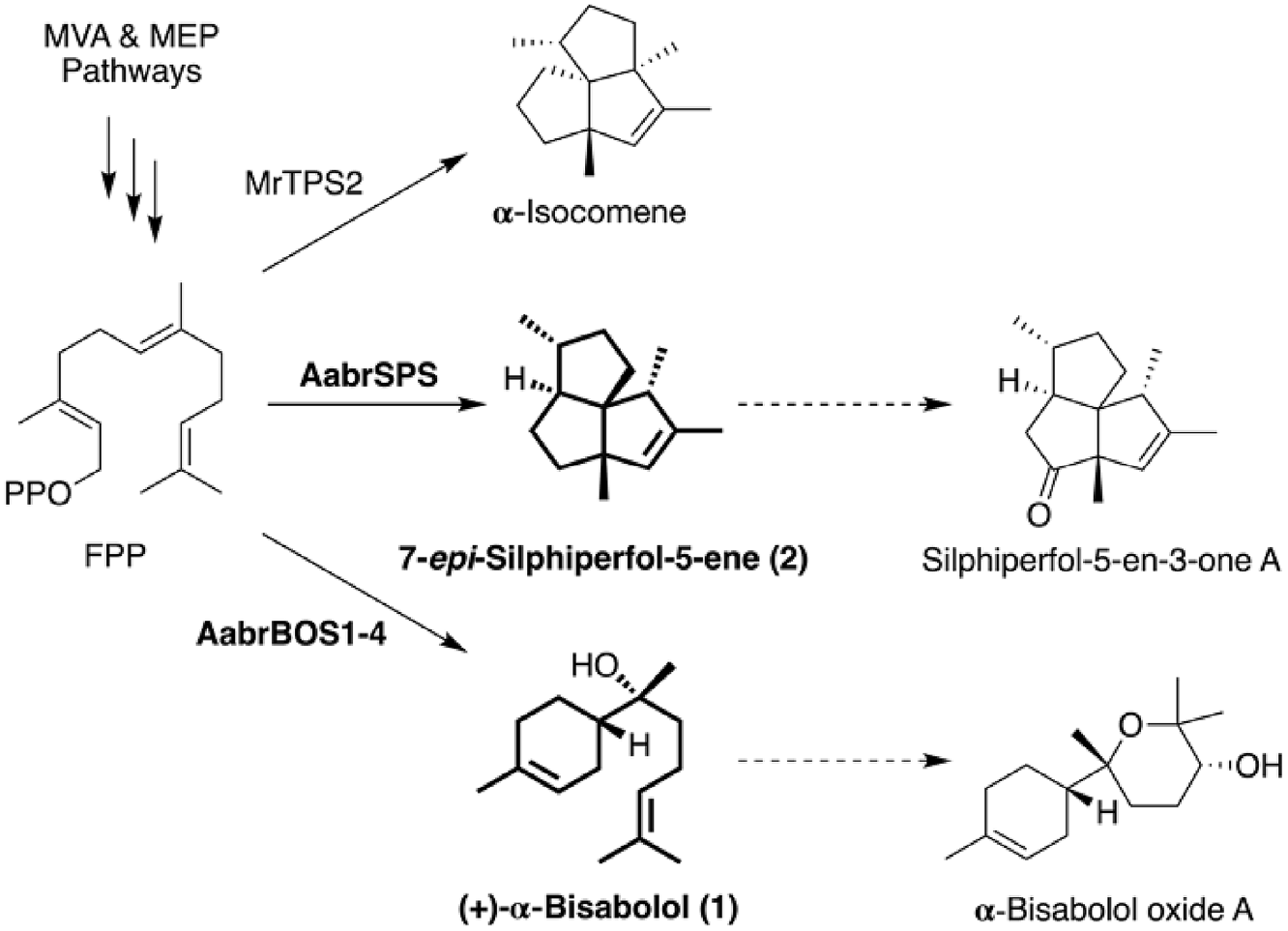
Sesquiterpenoid profiles of two different strains of A. abrotanum were analyzed. GC-MS analyses of n- hexane extracts of the aerial parts of A. abrotanum revealed 15 sesquiterpenoids, tentatively identified based on comparisons of retention indices and mass spectra with those of authentic compounds and reference data ( Fig. S1 View Fig and Table 1 View Table 1 ; Adams, 2007). β- Caryophyllene, germacrene D, bicyclogermacrene, and silphiperfolane-type sesquiterpenoids have been previously identified in A. abrotanum ( Obistioiu et al., 2014; Radulovi ć et al., 2009; Wang et al., 2018), whereas detection of β- and δ- elemenes, α- and βcopaenes, and α- humulene in this study is the first report of these compounds in A. abrotanum . Among all detected compounds, tricyclic silphiperfol-5-en-3-one A occupied 60% of the total sesquiterpenoid content of the extract. This finding strongly supported that the as yet unidentified gene encoding silphiperfolene synthase is highly expressed in the A. abrotanum used in this study.
Adams, R. P., 2007. Identification of Essential Oil Components by Gas Chromatography / Mass Spectrometry, fourth ed. Allured Publishing Corporation, Illinois.
Obistioiu, D., Cristina, R. T., Schmerold, I., Chizzola, R., Stolze, K., Nichita, I., Chiurciu, V., 2014. Chemical characterization by GC-MS and in vitro activity against Candida albicans of volatile fractions prepared from Artemisia dracunculus, Artemisia abrotanum, Artemisia absinthium and Artemisia vulgaris. Chem. Cent. J. 8, 6. https: // dx. doi. org / 10.1186 / 1752 - 153 X- 8 - 6.
Wang, Y., Li, X., Jiang, Q., Sun, H., Jiang, J., Chen, S., Guan, Z., Fang, W., Chen, F., 2018. GC-MS analysis of the volatile constituents in the leaves of 14 compositae plants. Molecules 23, 166. https: // dx. doi. org / 10.3390 / molecules 23010166.
Fig. 1. Proposed biosynthetic pathways of silphiperfol-5-en- 3-one A and (+)-α-bisabolol in A. abrotanum. The enzymes responsible for each step are indicated with arrows. The full names of the enzymes are given in the text. Determined biosynthetic pathways in this study are highlighted in bold. The α-bisabolol oxide A, detected by Radulović et al. (2009) and the previously reported sesquiterpene synthase synthesizing triquinane α-isocomene (MrTPS2; Irmisch et al., 2012) are also included. Dot arrows indicate a multi-step conversion.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.