Aegialomys galapagoensis ( Waterhouse, 1839 )
|
publication ID |
https://doi.org/ 10.1093/mspecies/sez013 |
|
publication LSID |
lsid:zoobank.org:pub:5F03FFB0-26FD-49BB-8C29-AFB1438044A2 |
|
persistent identifier |
https://treatment.plazi.org/id/0383FA74-AC26-FFE2-9EB4-FBCBFBF24906 |
|
treatment provided by |
Felipe |
|
scientific name |
Aegialomys galapagoensis ( Waterhouse, 1839 ) |
| status |
|
Aegialomys galapagoensis ( Waterhouse, 1839) View in CoL
Galapagos Rice Rat
Mus galapagoensis Waterhouse, 1839:66 View in CoL . Type locality “Chatham Island [= San Cristóbal Island], Galapagos Archipelago, Pacific Ocean,” Ecuador.
Hesperomys galapagoensis: Wagner, 1843:517 . Name combination.
Hesperomys (Oryzomys) galapagoensis: Thomas, 1884:453 . Name combination. Part.
Oryzomys bauri Allen, 1892:48 . Type locality: “Barrington Island [= Santa Fé Island],” Galapagos Islands, Ecuador.
O [ryzomys]. galapagoensis: Thomas, 1894:354 . Name combination.
[ Oryzomys View in CoL ] Bauri: Trouessart, 1897:527 . Name combination.
[ Oryzomys (Oryzomys) ] galapagoensis: Trouessart, 1904:419 . Name combination.
[ Oryzomys (Oryzomys) ] bauri: Trouessart, 1904:419 . Name combination.
Oryzomys galapagensis: Gyldenstolpe, 1932:23 . Incorrect subsequent spelling of Oryzomys galapagoensis ( Waterhouse, 1839) View in CoL .
[ Aegialomys View in CoL ] galapagoensis: Weksler, Percequillo, and Voss, 2006:5 View in CoL . First use of current name combination.
CONTEXT AND CONTENT. Context as for genus. No subspecies are currently recognized ( Pardiñas et al. 2017; Prado and Percequillo 2018).
NOMENCLATURAL NOTES. The common name of Aegialomys galapagoensis is Galapagos rice rat, or in Spanish, Rata Costera de Galápagos. The generic name is composed of the Greek word aegialos, in reference to its coastal distribution ( Weksler et al. 2006). Waterhouse (1839) described galapagoensis under the genus Mus , a genus taxon name that at that historic time (early 19th century) assembled most of the rat-like muroid rodents. Later, galapagoensis was transferred to the genus Hesperomys , a genus commonly employed for several species groups of South American cricetids (see Cabrera 1961). Later, Thomas (1884) assigned galapagoensis to Oryzomys , a subgenus of Hesperomys , and after that Allen (1892) moved Oryzomys to the generic rank, during the description of a new species from the Galapagos Archipelago, O. bauri (here assigned as a synonym of A. galapagoensis ). This scenario remained unchanged for more than a century, upon the allocation of the taxa of the species group name ( galapagoensis and bauri ) to a newly described genus Aegialomys by Weksler et al. (2006).
DIAGNOSIS
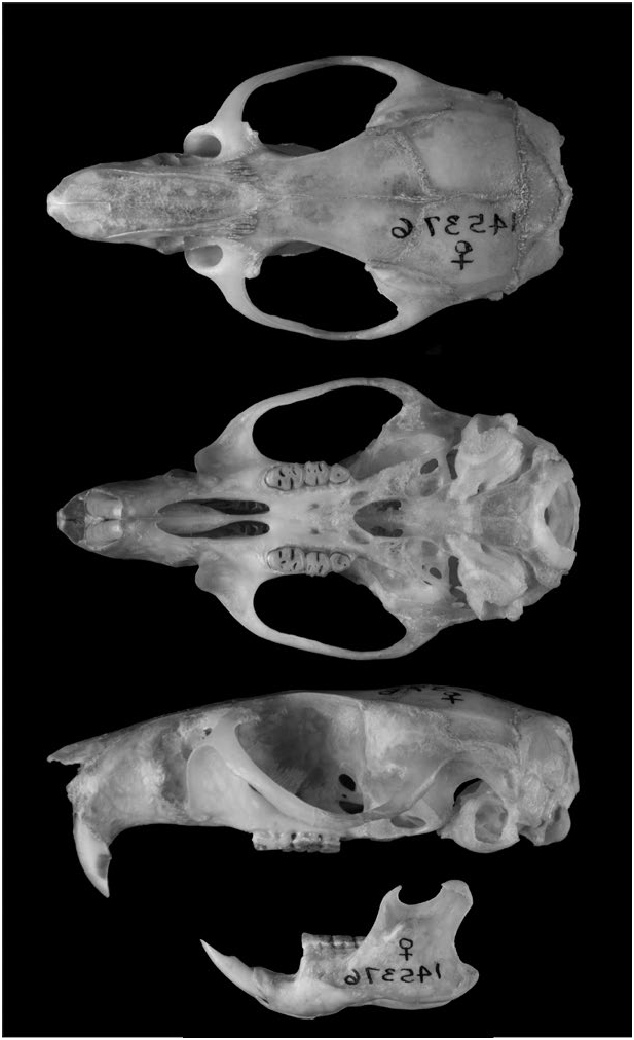
Aegialomys galapagoensis can be distinguished from others species of Aegialomys by its very long, dense, and lax pelage. Its viliform, setiform, and aristiform hairs are much longer compared to the other three species of Aegialomys , with modal length about 13, 20, 29 mm, respectively. The other three species of Aegialomys present a tail length larger than the head–body length, while A. galapagoensis exhibits a tail as long as the head–body length in most specimens, although some specimens present a tail length slightly shorter or a little longer than the head–body length, but not as long as the other species; the tail is also bicolored dorsal-ventrally, as in the other species of Aegialomys (Prado and Percequillo 2018; Fig. 1 View Fig ). The skull is large and robust ( Fig. 2 View Fig ), with one of the greatest overall size of skull in the genus (occipitonasal length range: 31.34–35.85 mm), only comparable to A. ica (occipitonasal length range: 28.76–38.39 mm; A. xanthaeolus occipitonasal length range: 26.38–35.05 mm, and A. baroni occipitonasal length range: 26.92–36.36—Prado and Percequillo 2018). A. galapagoensis exhibits the more robust upper molar series (mean length of molars, LM = 5.53 mm, range: 5.35–5.68; mean breadth of the first upper molar, BM1 = 1.74, range: 1.60–1.87) when compared to the other species of Aegialomys ( A. xanthaeolus, LM = 4.81 mm, range: 4.23–5.31, BM1 = 1.41 mm, range: 1.22–1.60; A. baroni, LM = 4.99 mm, range: 4.34–5.71, BM1 = 1.52 mm, range: 1.32– 2.02; and A. ica, LM = 5.42 mm, range: 4.52–6.09, BM1 = 1.64 mm, range: 1.39–1.95). The mesolophid in the lower molars m1 and m2 is always present in A. galapagoensis , while in A. baroni , A. ica , and A. xanthaeolus , this character can be found only in less than 25% of the individuals.
extending over three ventral scales. Tail scales are generally dark and about 20 per cm; tail bicolor (dark above and light below). Pinnae are long and densely haired on internal and external surfaces. Vibrissae moderately dense, not reaching beyond ears. Mystacial vibrissae not extending posteriorly beyond the caudal margins of the pinnae when laid back, and superciliary vibrissae not extending posteriorly beyond pinnae. Although no sexual dimorphism in Aegialomys was found by Prado and Percequillo (2011, 2018), Clark (1980) found significant differences in body size and weight with males heavier and with longer head–body length than females.
DISTRIBUTION
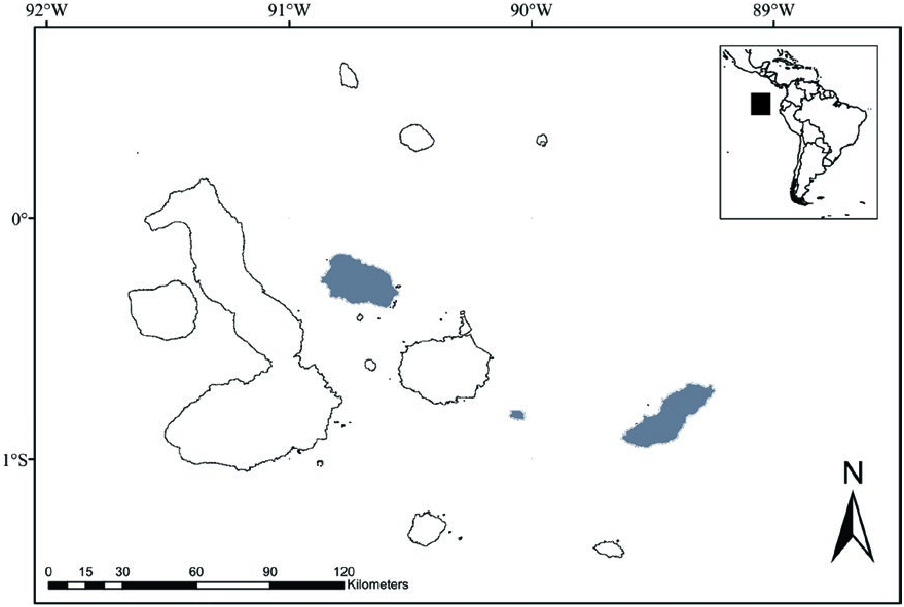
Known localities for Aegialomys galapagoensis occur on three islands of the Galapagos Archipelago, Santa Fé Island (Barrington Island), San Cristóbal Island (Chatham Island), and Santiago Island (James Island). Prado and Percequillo (2018) presented evidence for the presence of this species in Santiago Island, but discussed the validity of this record, based on specimens from the Natural History Museum (London, United Kingdom). These authors suggest that one specimen may have been mislabeled, being originally from San Cristóbal Island; the other specimen was more likely obtained at Santiago Island. The population of A. galapagoensis in San Cristóbal Island has been considered extinct since the early 1900s ( Heller 1904): recent surveys performed by Patton and Hafner (1983) and Dowler et al. (2000) recovered no specimens on this island ( Fig. 3 View Fig ).
GENERAL CHARACTERS
Aegialomys galapagoensis is the largest species in the genus ( Percequillo 2015; Pardiñas et al. 2017; Prado and Percequillo 2018), similar in size to A. ica , a species from Southern Peru (Prado and Percequillo 2018). It is a medium-sized rodent (total body length: 203–350 mm) characterized by very long, dense, and soft pelage. Dorsal coloration yellow or copper weakly grizzled; ventral pelage grayish-yellow ( Fig. 1 View Fig ). Pelage is a combination of short (11–13 mm) viliform hairs, long (18–20 mm) wavy setiform hairs, and very long (27–29 mm) and wide aristiform hairs. Tail length (range: 136–165 mm) as long as or slightly shorter or slightly longer than head–body length, and slightly pilose with hairs on dorsal and ventral regions, apparently
FOSSIL RECORD
Steadman et al. (1991:131, table 4) reported fossils of Aegialomys galapagoensis on San Cristóbal Island, associated with a Holocene vertebrate fauna with an estimated date range of 8,500–500 years before present.
FORM AND FUNCTION
Form. — The external morphology of Aegialomys galapagoenis follows the general pattern typical of Aegialomys . Digits II to V of the manus and pes have ungual tufts that are longer than claws, and dI has ungual tufts as long as claws. The length of claws is about 1 mm in the manus and about 2.5 mm in the pes. The pes is long and wide, dorsally covered with white hairs, with dI and dV smaller than the three central digits. The claw of dI extends slightly beyond one-half the length of the third phalanx of digit II, and digit V with claw extending to base of second phalanx of digit IV. The pes has six plantar pads, four interdigital at base of digits, one thenar, and one hypothenar (Prado and Percequillo 2018).
Skull is robust and strongly built. Rostrum is relatively long and wide (nasal length range: 11.76–15.09 mm; rostrum breadth range: 5.6–6.92 mm); zygomatic notch deep and wide; lacrimal small and in contact with frontal and maxillary. The zygomatic arches are robust, divergent posteriorly, wider near the zygomatic root (zygomatic width range: 16.25–19.78 mm). The interorbital region is strongly divergent posteriorly, with supraorbital margins sharp and acute, forming developed crests (interorbital width range: 4.92–5.91 mm). The braincase is elongated with a marked temporal bead. The parietals expand over the lateral surface of temporal region; lambdoidal and occipital crests are sharp. Rostrum with nasal projected anteriorly, greatly surpassing premaxillary and incisors. Jugal is present. The stapedial foramen is very small or absent (in some specimens, it is not discernible in the suture of the ectotympanic and petrosal; this latter condition was not mentioned by Voss 1988, but reported also for other genera of the tribe Oryzomyini , Cerradomys , Drymoreomys , and Sooretamys by Percequillo et al. 2008, Percequillo et al. 2011, and Chiquito et al. 2014, respectively). Carotid circulatory pattern 3 is present ( Voss 1988; Carleton and Musser 1989; Weksler 2003). The basicranial flexion is weakly pronounced with foramen magnum oriented caudally. Rostrum with incisive foramen long (occupying most of the diastema), with lateral margins wider medially and anteroposterior margins rounded, configuring a long and convex foramen. The posterior margins of incisive foramina penetrate between first molars in most specimens (ca. 36% in A. galapagoensis , 4% in A. xanthaeolus , 13% in A. baroni , and 48% in A. ica — Prado and Percequillo 2018), or leveled with the alveoli of first molars, or almost reaching the alveoli (length of incisive foramen range: 6.42–7.59 mm, width of incisive foramina range: 2.23–2.71 mm); posterior margin of zygomatic plate anterior to M1; palate intermediate (sensu Weksler 2003) with palatal bridge flat (palatal bridge length range: 5.41–6.55 mm, palatal width range: 6.37–7.60 mm). The mesopterygoid fossa has an anterior margin that is variable in shape and extending above the maxillary bones. Auditory bullae inflated, with short and wide Eustachian tube. Mandible is robust, the ramus deep, with coronoid and condyloid processes well developed. ( Fig. 2 View Fig ; Prado and Percequillo 2018).
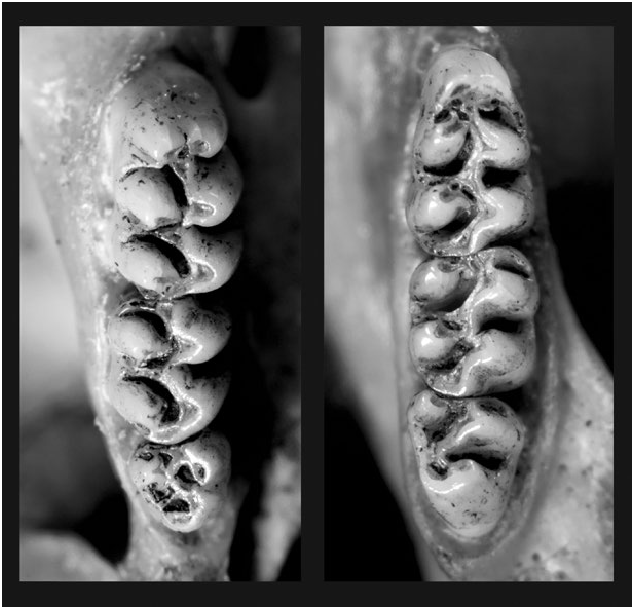
Upper molar series robust, long, and wide. First upper molar with anterocone divided by anteromedian flexus in about 27% of specimens analyzed by Prado and Percequillo (2018), first lower molar with anteroconid undivided; mesoloph present in the first and second molars; mesolophid present in the first molar in all specimens, and in the second molar in 78% of specimens analyzed by Prado and Percequillo (2018; Fig. 4 View Fig ).
Fifth lumbar vertebra with well-developed anapophysis; hemal arch between second and third caudal vertebrae with spiny posterior process; hemiglandular and unilocular stomach without extension of glandular epithelium in the corpus. The phallus presents glans penis complex, with small distal and trifurcated bacular cartilage (with a central, short and thin digit), a pair of preputial glands present, smooth tissues (not spiny) on margin of terminal crater rim do not hide bacular projections; spineless dorsal papilla; urethral processes without subapical lobes; the phallus is elongated, with a length/diameter ratio of 1.8. The male accessory reproductive glands consist of a pair of preputials, bulbourethral, ampullary, vesiculars, and four pairs of prostate, very similar to other oryzomyines (Patton and Hafner 1983).
Function. —The shape of the hindfeet, assessed by length and width, and the length of the claws along with the very large and fleshy plantar pads (thenar and hypothenar pads large and distinct), with interdigital pads set close together, suggest that Aegialomys galapagoensis is a terrestrial species with some scansorial abilities.
The current concept of the tribe Oryzomyini includes pentalophodont, typical forest-specialist genera and also tetralophodont, open-dweller genera (sensu Weksler et al. 2006). Some genera in the tribe are transitional habitat specialists, such as Microakodontomys , Oligoryzomys ( Hershkovitz 1993) , and Cerradomys ( Percequillo et al. 2008) , that exhibit polymorphism regarding the mesoloph, which is reduced or absent in some individuals or species. Similarly, A. galapagoensis is also a specialist in open, transitional habitats (halophytic vegetation mixed with cacti forest) and as such exhibits reduced or absent mesolophid in some specimens. Therefore, there is a wellknown correlation between the presence of the mesoloph and mesolophid and habitat, although there is not a clear relation on their form and function.
ONTOGENY AND REPRODUCTION
There is no sexual dimorphism during age development, and the ontogenetic variation described for Aegialomys galapagoenis follows the pattern described for A. xanthaeolus (Prado and Percequillo 2011) and other rodents of the tribe Oryzomyini (see Carleton and Musser 1989; Voss 1991): the variation is larger in measurements related to craniofacial and incisor components, as these have more conspicuous growth related to ontogenetic development. But the dimensions of the molars and the measurements of the neurocranium, which complement the growth in the early postnatal life ( Voss 1991), are relatively less variable.
The sex ratio of A. galapagoenis was biased toward males, as males were trapped significantly more than females ( Clark 1980). Dowler et al. (2000) recovered similar results, with a sex ratio favoring males (1.3:1). Harris and MacDonald (2007) also recovered a bias toward males in the Santiago Galapagos mouse Nesoryzomys swarthi , another Galapagos endemic oryzomyine rodent. They hypothesized that transient males caused this asymmetry, as males and females exhibit similar survival rates, although males present a lower interannual capture rate. Harris and MacDonald (2007) concluded that transient males could also explain the sex ratio biased towards males in A. galapagoensis , and added, that home range sizes in males are larger in both species.
According to Clark (1980), reproduction occurs in the hot season, with young individuals collected in May and March, while Brosset (1963) collected young specimens in March, July, and December. Sampling in August 1995, Dowler et al. (2000) observed no lactating females, but reported males with scrotal testis (11 individuals of 27). The number of embryos per female was 2–7 ( Brosset 1963; Clark 1980), and it was suggested that embryo production is limited by the amount of rainfall which is associated with food availability ( Clark 1980; Harris and MacDonald 2007). Harris and MacDonald (2007:216) stated “females adjust their reproductive output according to resource availability, perhaps through failed implantation or embryo reabsorption.” Brosset (1963) revealed that the young are born completely naked and blind; they develop fur and open their eyes from day 4 to day 9; adult pelage starts appearing at day 13. The dynamics of body size revealed that there were two recruitment periods throughout the year: young individuals appear during the warmer season, coupled with the presence of many large year-old rats. In the nonbreeding season, young rats grow and old rats die, compressing the size distributions toward normality ( Clark 1980).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Aegialomys galapagoensis ( Waterhouse, 1839 )
| Prado, Joyce R & Percequillo, Alexandre R 2019 |
Oryzomys galapagensis:
| GYLDENSTOLPE, N. 1932: 23 |
Oryzomys (Oryzomys)
| TROUESSART, E. L. 1904: 419 |
Oryzomys (Oryzomys)
| TROUESSART, E. L. 1904: 419 |
Oryzomys
| TROUESSART, E. L. 1897: 527 |
Oryzomys bauri
| ALLEN, J. A. 1892: 48 |
Hesperomys (Oryzomys) galapagoensis:
| THOMAS, O. 1884: 453 |
Hesperomys galapagoensis:
| WAGNER, J. A. 1843: 517 |
Mus galapagoensis
| WATERHOUSE, G. R. 1839: 66 |