Centrophorus uyato ( Rafinesque, 1810 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5155.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D13EBD69-7110-44F7-AEFA-04582EE21551 |
|
DOI |
https://doi.org/10.5281/zenodo.6674838 |
|
persistent identifier |
https://treatment.plazi.org/id/038487D1-FFA3-FFBF-BEE1-CF0B3DD4FDCF |
|
treatment provided by |
Plazi |
|
scientific name |
Centrophorus uyato ( Rafinesque, 1810 ) |
| status |
|
Centrophorus uyato ( Rafinesque, 1810) View in CoL
Little Gulper Shark
Synonymy.
Dalatias nocturnus Rafinesque, 1810: 11 , pl. 14, fig. 3 (Type locality: Sicily, Italy)— Cuvier, 1818: 455; Cuvier, 1829: 392; Cuvier, 1837: 246; Swainson, 1838: 129, 160; Swainson, 1839: 313; Duméril, 1865: 436; Jordan & Evermann, 1917: 77; Bigelow & Schroeder, 1948: 500.
Spinax uyatus — Bonaparte, 1834: 49, fig. 2 ( Italy); Bonaparte, 1846: 16 (Mediterranean); Böhlke, 1984: 158.
Acanthias uyatus — Müller & Henle, 1839: 85 (Mediterranean); Gray, 1851: vii, 71 (Mediterranean); Steindachner, 1864: 27; Duméril, 1865: 439 (Algerian coast, Mediterranean); Barbosa du Bocage & de Brito Capello, 1866: 7, 21; Günther, 1870: 419 (Mediterranean); Canestrini, 1872: 40; Steindachner, 1875: 466; Moreau, 1881: 346 (Mediterranean); Réguis, 1882: 72; Rochebrune, 1883: 47 ( Senegal and Gambia); Hilgendorf, 1884: 518; Duncan, 1891: 35 (Mediterranean); Moreau, 1892: 39; Seeley, 1895: 35 (Mediterranean); Parona, 1898: 38 (Ligurian Sea); Duncker, 1914: 291; de Buen, 1916: 303, figs (Mediterranean coast of Morocco); Landolt, 1947: 353.
Centrophorus granulosus View in CoL — Müller & Henle, 1839: 89, pl. 33 (Sicily, Italy); Barbosa du Bocage & de Brito Capello, 1864: 261 ( Portugal); Vinciguerra, 1883: 18 (482) (Gulf of Genoa); Goode & Bean, 1896: 12, pl. 3, fig. 11 (W Atlantic, Mediterranean and adjacent areas); Boutan, 1926: 1 ( Algeria); Dieuzeide, 1928a: 15, figs ( Algeria); Dieuzeide, 1928b: 1314 ( Algeria?); Andr & Canal, 1929: 511 ( Algeria); Arcidiacono, 1931: 609 (Gulf of Squillace, Italy); Gruvel, 1931: 74 (State of Syria [ Syria, Lebanon, Israel]); Ranzi, 1932: 240 (Naples, Italy); Belloc, 1934: 146, fig. ( Western Sahara, Morocco, Canary Islands and Madeira); Ranzi, 1934: 343, 370 (Naples, Italy); Fowler, 1936: 73 (Mediterranean); Fowler, 1941 (in part): 231 (Mediterranean, inc. Italy); Šoljan, 1948: 66, figs (Adriatic Sea); Bigelow et al., 1955: 6 (Gulf of Mexico); Kirinčić & Lepetić, 1955: 24 (southern Adriatic Sea); Tortonese, 1956: 176, figs 94-95 ( Italy); Cadenat, 1959: 748, fig. 1b (West Africa); Maurin, 1968: 82 ( Morocco to Algeria); Maurin & Bonnet, 1970: 147 (Canary Islands to Cape Verde); Karrer, 1975: 64 ( Namibia); Karlovac, 1976: 601, fig. 4 (Adriatic Sea); Guitart, 1979: 67, fig. 46 ( Cuba); Bouchet et al., 1982: 577 ( Tunisia); Zupanovic & El-Buni, 1982: 111 ( Libya); Uyeno et al., 1983: 62, figs ( Suriname); Compagno, 1984a: 37, figs (Atlantic and Indo-West Pacific); Gilat & Gelman, 1984: 263 (Levant Sea, Israel); McEachran & Branstetter, 1984: 130, fig. (NE Atlantic and Mediterranean); Muñoz-Chapuli, 1984: 9 (NE Atlantic);? Quéro, 1984: 43, fig.; Capapé, 1985: 97, fig. 1–7 (Eastern Atlantic); Jardas, 1985: 50 (Adriatic Sea); Anon, 1986: 93, fig. 22 (Atlantic Ocean); Bass et al., 1986 (in part): 50, fig. 5.1 ( Walvis Bay and Mozambique); Golani, 1986: 23, fig. 2 (Levantine Sea, Israel); Muñoz-Chapuli & Ramos, 1989: 65, figs 1a, 3a, 4b and c, 5a, 6a, 7a (NE Atlantic and Mediterranean); Compagno et al., 1991: 54 (Hondeklip Bay to Namibia); Benli et al., 1993: 133, figs 1 and 2 (Sea of Marmara); Pisanty & Golani, 1995: 388 (Levantine Sea, Israel); Lanfranco, 1996: 6, pl. 4 ( Malta); Aldebert, 1997: 284 (Gulf of Lion, France); Guallart, 1998: 1, figs (Balearic Sea); Bello, 1999: 69 (Adriatic Sea); Hernández-Hamón & Núñez, 1998: 107 (Pacific Columbia); Ungaro et al., 1999: 180 ( Albania); Capapé et al., 2000: 129 (Languedoc coast, France); Bertrand et al., 2000: 5 ( Gibraltar to Aegean Sea, Mediterranean); Golani & Pisanty, 2000: 71 (Levantine Sea, Israel); Baino et al., 2001: 234 (Alboran Island to Aegean Sea, Mediterranean); Guallart & Vicent, 2001: 135, fig. 4 (Balearic Sea, Spain); Bilecenoglu et al., 2002: 16 ( Turkey); Schembri et al., 2003: 76, fig. 3c ( Malta); McLaughlin & Morrissey, 2004: 481, fig. 3 (Cayman Trench, Jamaica); Moreno García, 2004: 214 (in part, not figs) (Mediterranean Sea); Sion et al., 2004: 155 (Ionian Sea, Greece); Golani, 2005: 11 (Levantine Sea, Israel); Lteif, 2015: 16, figs 9a, 14, ( Lebanon); Serena, 2005: 27, figs, pl. I, 8 (Mediterranean Sea); Serét, 2005: 21 ( Libya); Bessho, 2006: 28, figs 27–30, 33–35 ( Japan, Namibia); Golani et al., 2006: 38, fig. (eastern Mediterranean); Hadjichristophorou, 2006: 163 ( Cyprus); Megalofonou & Chatzispyrou, 2006: 67, fig. 4 (Crete, Greece); Mejía-Falla et al., 2007: 116 ( Colombia); Psomadakis et al., 2009: 200 (Gulf of Naples, Italy); D’Onghia et al., 2010: 401 (Ionian Sea, Greece); Lipej & Dulĉić, 2010: 10 (Adriatic Sea); Damalas & Vassilopoulou, 2011: 145 (Aegean Sea, Greece); Colloca & Lelli, 2012: 12 ( Lebanon); Costa et al., 2012: 7 ( Portugal); Guijarro et al., 2012: 89 (Balearic Islands, Spain); Güven et al., 2012: 278 (Antalya Bay, Turkey); Iglésias, 2013: 38, pl. 16 ( France and Cape Verde); Carneiro et al., 2014: 13 ( Portugal); Farrugio & Soldo, 2014: 33 (Sicily, Italy and Tunisia); Veríssimo et al., 2014: 6 (Gulf of Mexico); Goren & Galil, 2015: 510 (Levant Sea, Israel); Barría et al., 2015a: 226 (Catalan Sea, Spain and Gulf of Lions, France); Barría et al., 2015b: 114 (Catalan Sea, Spain and Gulf of Lions, France); Carpenter & De Angelis, 2016: 1170, figs (Eastern Atlantic); Ramírez-Amaro et al., 2016: 639 (western Mediterranean); Cariani et al., 2017: 5 (Mediterranean);? Haroun et al., 2017: 84 ( Egypt); Gajić, 2019: 101, fig. 14 ( Croatia, Montenegro and Albania); Bariche & Fricke, 2020: 17, fig. 12 ( Lebanon).
? Acanthias nigrescens Nardo, 1860: 70 , 96 (Type locality: Venice, Italy).
Entoxychirus uyatus — Gill, 1862: 498; Whitley, 1934: 199 ( Australia).
Acanthias ujatus — Döderlein, 1878: 30 (Sicily, Italy); Döderlein, 1881: 92 ( Italy).
Centrophorus uyatus View in CoL — Goode & Bean, 1896: 508; Garman, 1906: 204; Garman, 1913: 9, 196.
Squalus uyatus — Garman, 1899: 28.
Squalus uyato — Garman, 1906: 204.
Centrophorus bragancae Regan, 1906: 438 View in CoL (Type locality: Cezimbra, Portugal)— Regan, 1908: 53 (coast of Portugal); Strand, 1908: 83.
Centrophorus uyato View in CoL — Fowler, 1936: 72, fig. 21 (Mediterranean and eastern Atlantic); Tortonese, 1938: 310 (Mediterranean); Poll 1951: 64, figs 33–34 ( Angola and Namibia); Bigelow et al., 1953: 227, fig. 4 (Gulf of Mexico; Nice, Mediterranean Sea); Aksiray, 1954?: 233 (Turkish Seas); Bigelow et al., 1955: 5, 9; Springer & Bullis, 1956: 42 (Gulf of Mexico); Bigelow & Schroeder, 1957: 54, 66, 69, 72, 79–84, fig. 8e (Gulf of Mexico); Springer & Garrick, 1964: 81, 91; Krefft & Tortonese, 1973: 39 (NE Atlantic and Mediterranean); Bass et al., 1976: 31, figs 22, 24E, 24F, pl. 7 (southern Mozambique); Bridger, 1978: 26 (west of Ireland and Britain); Compagno, 1981: 8 [sharks] (Eastern Central Atlantic); Castro, 1983: 54, figs (Gulf of Mexico); Allu et al., 1984: 125 ( Namibia); Compagno, 1984a: 45, figs (Atlantic, Indo-West Pacific); Compagno, 1984b: 9 [sharks] (Western Indian Ocean ); McEachran & Branstetter, 1984: 132, fig. (NE Atlantic and Mediterranean); Lloris, 1986: 96, fig 24 ( Namibia); Turón et al., 1986: 63 ( Namibia); Fischer et al., 1987: 823 (Mediterranean); Compagno, 1988b: 603 ( Comoro Islands); Compagno et al., 1989: 24, fig. (Hondeklip Bay, South Africa to Namibia);? Clark & Kristof, 1990: 277, fig. 9 (Caribbean); Springer, 1990: 11 (Gulf of Mexico, Mediterranean, and Natal, South Africa); Applegate et al., 1993: 35 (Atlantic Mexican waters); Gomon et al., 1994: 92, 94, figs 30, 31 (southern Australia); Last & Stevens, 1994: 60, figs, pl. 4 (fig. 8.5) (southern Australia); Meriç, 1995: 192 (Sea of Marmara, Turkey); Perry et al., 1995: 139 (Gulf of Mexico); Reiner, 1996: 22, fig. ( Cape Verde); Bonfil, 1997: 105 (Veracruz, Mexico); Joseph, 1999: [unpaginated] ( Sri Lanka); Cervigón & Alcalá, 1999 ( Venezuela); Clarke, 2000: 377 (Rockall Trough, NE Atlantic); Baino et al., 2001: 234 (Alboran Island to Aegean Sea, Mediterranean); Graham et al., 2001: 551 (south-eastern Australia); Yearsley et al., 2001: 35, 360 (southern Australia); Bilecenoglu et al., 2002: 17 ( Turkey); Daley et al., 2002: 53 (southern Australia); Ali & Saad, 2003: 58, fig. ( Syria); Schembri et al., 2003: 77, fig. 3d ( Malta); Serena, 2005: 28, figs, pl. I, 9 (Mediterranean Sea, NE Atlantic, Western Indian, Gulf of Mexico and Taiwan); Meriç et al., 2007: 31 ( Turkey); White, 2008: 87, figs (southern Australia); Scacco et al., 2010: 39, fig. 3f (Mediterranean Sea); Castro, 2011: 81, figs 16a-e (north-western Atlantic); Davenport et al.: 2011: 557 (north-western Atlantic, USA); White et al., 2013: 36, fig. 2, 15, 16 (Atlantic); Veríssimo et al., 2014: 6, fig. 5 (Gulf of Mexico); Hipes, 2015: 1, fig. 11 (Gulf of Mexico, USA); Wienerroither et al., 2015: 834, fig. 2 (northern Norway); Farrag, et al., 2016: 481, fig. 2a ( Egypt); Driggers et al., 2017: 52 (Gulf of Mexico); Haroun et al., 2017: 84 ( Egypt); Lteif et al., 2017: 1491 ( Lebanon); Biscoito et al., 2018: 471, fig. 7 (Madeira, Portugal); Carneiro et al., 2019: 36 ( Portugal); Ehemann et al., 2019: 4 ( Venezuela); Fernando et al., 2019: 231, figs 5e, 18d-f (Mutur, Sri Lanka); Follesa et al., 2019: 85 (Mediterranean); Psomadakis et al., 2019: 162, figs, pl. X (fig. 70) ( Myanmar); Iglésias, 2020: 46, pl. 21 ( France and Cape Verde); Ebert & Dando, 2021: 217, figs (NE Atlantic and Mediterranean); Kousteni et al., 2021: 1, figs 2 and 3 (Cypriot waters).
Centrophorus machiquensis Maul, 1955: 5 View in CoL , figs 13–16 (Type locality: Madeira)— Krefft & Tortonese, 1973: 39 (Madeira); Ali & Saad, 2003: 57, fig. ( Syria); Biscoito et al., 2018: 470, fig. 6 (Madeira, Portugal); Almeida & Biscoito, 2019: 99 (Canary Islands and Madeira); Carneiro et al., 2019: 36 ( Portugal).
Centrophorus ujato — Tortonese, 1956: 178, fig. 96 (Genova, Italy); Bini, 1967: 97, fig. ( Italy); Sara, 1968: 1, figs 1–3 (west of Sicily); FAO, 1971: no pagination (Mediterranean Sea).
Centrophorus spp. ( granulosus View in CoL group)— Forster et al, 1970 (in part): 388 (Western Indian Ocean ).
Centrophorus (forme) uyato-machiquensis — Cadenat & Blache, 1981: 58, figs. 36, 37 and 40 (NE Atlantic).
Centrophorus spp. — Peyronel et al., 1984: 643 (Bay of Ajaccio, Corsica, France).
Centrophorus cf. harrissoni View in CoL (Undescribed gulper shark #2)— Kiraly et al., 2003: 2 ( Puerto Rico, US Virgin Islands, Virginia, North Carolina and Florida Straits to Dry Tortugas).
Centrophorus bragance — Hernández-Hamón & Núñez, 1998: 108 (as questionable synonym of C. granulosus View in CoL ).
Centrophorus sp. ( uyato View in CoL ?)— Morón et al., 1998: 144 (Beruwela, Sri Lanka).
Centrophorus sp. cf. uyato View in CoL — Saad, et al., 2004: 430 ( Syria).
? Centrophorus sp. (non uyato View in CoL )— Serét, 2005: 21 ( Libya).
Centrophorus zeehaani White, Ebert & Compagno, 2008: 1 View in CoL , figs 8-10 (Type locality: South Australia)— Last & Stevens, 2009: 68, pl. 9.7, figs (southern Australia); Pethybridge et al., 2010: 1369 (Tasmania and Great Australian Bight, Australia); Pethybridge et al., 2011: 2743 (Tasmania and Victoria, Australia); Graham & Daley, 2011: 583 (southern Australia); Daley et al., 2012: 708 (southern Australia); White et al., 2013: 41 (southern Australia); Daley et al., 2015:127 (southern Australia); Wienerroither et al., 2015: 834, fig. 2 (northern Norway); Bineesh et al., 2016: 461 (Kollam, India).
Centrophorus cf. uyato View in CoL — McLaughlin & Morrissey, 2005: 1185, figs 2, 3 (Cayman Trench, Jamaica); Veríssimo et al., 2014: 7 (Gulf of Mexico); Serena et al., 2020: 502, 509 (Mediterranean); Bellodi et al., 2022: 2 (Mediterranean Sea).
Centrophorus zeehani — Daley et al., 2012: fig. 2 (misspelling; southern Australia); Bineesh et al., 2016: 466 (misspelling; Kollam, India).
Centrophorus ‘uyato’ View in CoL — White et al., 2017: 86 (Eastern Atlantic); Almeida & Biscoito, 2019: 100 (Mediterranean Sea, Canary Islands and Madeira).
Centrophorus cf. granulosus View in CoL — Follesa et al., 2019: 85 (Mediterranean); FAO, 2018: unpaginated, fig. (Mediterranean Sea).
? Centrophorus granulosus View in CoL — Parenti, 2019: 102 (Sicily).
Material examined. Neotype: BMNH 2021.10.4.1 (eviscerated; GenBank accession ON167716 View Materials ), female 983 mm TL, between Gorgona and Capraia islands, Ligurian Sea, 43°19.8′ N, 9°56.1′ E, 180 m, 20 Dec. 2012.
Other specimens: Australia: AMS I 44310 View Materials –001 (paratype of Centrophorus zeehaani ; GenBank accession ON167706 View Materials ), adult male 826 mm TL, southwest of Coffin Bay, South Australia, 35°14′ S, 134°29′ E, 360–600 m, 28 July 2005; CSIRO CA 4104, adult male 843 mm TL, east of Gabo Island, Victoria, 37°40′ S, 150°15′ E, 504–508 m, 4 May 1984; CSIRO H 866–02, immature male 456 mm TL, CSIRO H 867–01, female 439 mm TL, east of Jervis Bay, New South Wales, 34°58′ S, 151°09′ E, 490–576 m, 10 Sep. 1986; CSIRO H 2268–02, adult male 800 mm TL, west of Bunbury, Western Australia, 33°03′ S, 114°25′ E, 701 m, 10 Feb. 1989; CSIRO H 6307–01 (skeletal parts), female 1027 mm TL, 12 July 2004, east of Flinders Island, Tasmania, ~ 40° S, ~ 149° E, 350–430 m; CSIRO H 6309–01 (skeletal parts; GenBank accession ON167708 View Materials ), adult male 865 mm TL, CSIRO H 6309–02 (skeletal parts), adult male 876 mm TL, CSIRO H 6309–04 (skeletal parts), adult male 906 mm TL, east of Flinders Island, Tasmania, ~ 40° S, ~ 149° E, 400–450 m, 1 Aug. 2004; CSIRO H 6310–04 (skeletal parts), female 970 mm TL, northeast of Flinders Island, Tasmania, 39°04′ S, 148°39′ E, 500–680 m, 24 Jul. 1986; CSIRO H 6311–01 (skeletal parts), female 655 mm TL, east of St. Helens, Tasmania, 41°27′ S, 148°44′ E, 850–860 m, 5 Jun. 1987; CSIRO H 6500-02, adult male 862 mm TL, east of Flinders Island, Tasmania, 40°15′ S, 148°45′ E, 329–512 m, 21 Aug. 2003; CSIRO H 6503–02 (skeletal parts), female 991 mm TL, CSIRO H 6503–03 (skeletal parts; GenBank accession ON167709 View Materials ), female 1023 mm TL, CSIRO H 6503–04 (skeletal parts; GenBank accession ON167710 View Materials ), female 987 mm TL, CSIRO H 6503–05 (skeletal parts), female 957 mm TL, northeast of Flinders Island, Tasmania, 39°20′ S, 148°45′ E, 370–420 m, 7 Apr. 2003; CSIRO H 6504–02, adult male 854 mm TL, CSIRO H 6504–03, female 817 mm TL, CSIRO H 6504–04, juvenile male 666 mm TL, CSIRO H 6504–05, adult male 861 mm TL, east of Jervis Bay, New South Wales, 35°12′ S, 150°58′ E, 320–500 m, July to Aug. 2003; CSIRO H 6628–01 (paratype of Centrophorus zeehaani ), immature male 506 mm TL, CSIRO H 6628–02 (paratype of Centrophorus zeehaani ; GenBank accession ON167705 View Materials ), immature male 645 mm TL, CSIRO H 6628–03 (paratype of Centrophorus zeehaani ), adult male 875 mm TL, CSIRO H 6628–04 (paratype of Centrophorus zeehaani ), adult male 910 mm TL, CSIRO H 6628–05 (holotype of Centrophorus zeehaani ), adult male 893 mm TL, CSIRO H 6628–06 (paratype of Centrophorus zeehaani ), adult male 852 mm TL, CSIRO H 6628–07 (paratype of Centrophorus zeehaani ), adult male 906 mm TL, NMV A 29736 View Materials –001 (paratype of Centrophorus zeehaani ), adult male 820 mm TL, southwest of Coffin Bay, South Australia, 35°14′ S, 134°29′ E, 360–600 m, 28 July 2005; CSIRO unreg. (DB 02/181), Great Australian Bight, adult male 857 mm TL; CSIRO unreg. (LJVC 880517), female 840 mm TL, unknown location; PMH095-11 (jaws only), female 104 cm TL, Albany, Western Australia.
Eastern Atlantic (including Mediterranean): AMNH 78267, female 922 mm TL, AMNH 78269, female 937 mm TL, AMNH 78271, female 1016 mm TL, AMNH 78273, adult male 872 mm TL, AMNH 78277, adult male 895 mm TL, AMNH 78279, adult male 890 mm TL, between Tenerife and Gran Canaria, Canary Islands, Spain, 3 Oct. 1986; AMNH 78280, female 996 mm TL, AMNH 78282, female 1059 mm TL, AMNH 78283, female 1056 mm TL, AMNH 78284, adult male 832 mm TL, AMNH 78285, female 921 mm TL, AMNH 78286, female 1004 mm TL, AMNH 78291, female 1034 mm TL, AMNH 78292, female 980 mm TL, AMNH 78294, adult male 891 mm TL, between Tenerife and Gran Canaria, Canary Islands, Spain, 4 Oct. 1986; BMNH 1862.4.22.29, female 449 mm TL, Madeira, Portugal; BMNH 1864.7.18.1, female 894 mm TL, Madeira, Portugal; BMNH 1904.11.30.9- 10 (2 specimens), female 1036 mm TL, adult male 888 mm TL, west of Faro, Portugal, 662 m depth; BMNH 1904.11.30.11 (paralectotype of Centrophorus bragancae ), juvenile male 488 mm TL, off Sesimbra, Portugal, 841 m depth; BMNH 1904.11.30.12 (lectotype of Centrophorus bragancae ), female 467.5 mm TL, off Sesimbra, Portugal, 505 m depth; BMNH 2013.9.20.34, female 875 mm TL, BMNH 2013.9.20.35, female 930 mm TL, BMNH 2013.9.20.36, female 935 mm TL, BMNH 2013.9.20.37, female 731 mm TL, BMNH 2013.9.20.38, adult male 843 mm TL, BMNH 2013.9.20.39, adult male 865 mm TL, northeast Atlantic; CSIRO H 7471-01 (GenBank accession ON167715 View Materials ), adult male 853 mm TL, east of Corsica, France, 42°05.8′ N, 9°44.5′ E, 550–565 m, 30 May 2012; CSIRO H 7472-01, juvenile male 440 mm TL, southeast of Corsica, France, 41°47.9′ N, 9°30.5′ E, 466–480 m, 31 May 2012; ERB 1288, male 770 mm TL, Concarneau fish-market, France, 9 Sep. 2000; ERB 0763, male, 850 mm TL, Concarneau, France, 13 May 2009; HUJ 10885, female 760 mm TL, Hadera, Israel, 13 Sep. 1982; HUJ 11339, adult male 796 mm TL, Haifa Bay, Israel, 1 Mar. 1983; HUJ 17029, female 476 mm TL, Haifa, Israel, 16 Mar. 1983; HUMZ 151304, juvenile male 578 mm TL; HUMZ 151306, female 978 mm TL, Namibia; HUJ 21135 (GenBank accession ON167718 View Materials ), female 753 mm TL, Haifa fishing port, Israel, 29 July 2013; MNHN-IC 1905- 0568, juvenile male 454 mm TL, Portugal, 460 m depth, June 1903; MNHN-IC 1969-0269, juvenile male 499 mm TL, southwest of Monrovia, Liberia, 6°08′ N, 10°56′ W, 400 m depth, 27 Apr. 1964; MNHN-IC 0000-1224, female 537 mm TL, Naples, Italy; MNHN-IC 2005-0169, adult male 892 mm TL, west of Ireland, 28 Apr. 2005; NMW 15009, female 889 mm TL, Nice; NMW 63020, adult male 880 mm TL, Nice, France, 1902; PMH095-13 (jaws only), male 860 mm TL, Lisbon fish auction, Portugal; PMH095-14 (jaws only), female 905 mm TL. Algeciras fish market, Spain; SBMC unreg (BPS-0488), female 670 mm TL, Cape Verde, 15°27.75’ N, 23°25.91’ W, 100–300 m depth, 16 Jun. 2005; ZMB 22737, female 456 mm TL, Naples, Italy; ZMB 22993, juvenile male 432 mm TL, South Atlantic Ocean; ZMH 120700, adult male 803 mm TL, northwest of Scotland, 57°40’ N, 09°35’ W, Apr. 1982, 680 m depth.
In addition, 506 specimens studied by Guallart (1998) from Balearic Sea (Western Mediterranean); only some samples and photographic material preserved.
Western Atlantic: AMNH 33442, adult male 857 mm TL, off Mississippi, Gulf of Mexico, USA, 24 Aug. 1962; AMNH 33443, juvenile male 539 mm TL, off Mississippi River mouth, Gulf of Mexico, USA, 29°11′ N, 88°08′ W, 20 Dec. 1962; CAS 60861, female 987 mm TL, south of St. Thomas Harbor, US Virgin Islands, 18°11′12” N, 64°55′30” W, 340–460 m depth, 22 Sep. 1987; PMH095-1 (jaws only), male 850 mm TL, northern Gulf of Mexico, USA; PMH095-2 (jaws only), male 855 mm TL, northern Gulf of Mexico, USA; PHM095-3 (jaws only), male 900 mm TL, northern Gulf of Mexico, USA; PHM095-4 (jaws only), male 910 mm TL, northern Gulf of Mexico, USA; PMH095-7 (jaws only), female 870 mm TL, northern Gulf of Mexico, USA; PMH095-8 (jaws only), female 875 mm TL, northern Gulf of Mexico, USA; PMH095-9 (jaws only), female 1010 mm TL, northern Gulf of Mexico, USA; PMH095-11 (jaws only), female 1080 mm TL, northern Gulf of Mexico, USA; PMH095-12 (jaws only), female 1090 mm TL, northern Gulf of Mexico, USA; SAM unreg (Oregon II 37238), female 500 mm TL, SAM unreg (Oregon II 11281), female 393.5 mm TL, Gulf of Mexico, USA; ZMH 119879, juvenile male 808 mm TL, east of Florida, USA, 29°08′ N, 78°57′ W, 4 Nov. 1979.
Western Indian Ocean : BMNH 1973.7.9.17, female 735 mm TL, east of settlement on Aldabra Island, Seychelles, 878 m depth, 7 January 1969; CAS 29287, female 1024 mm TL, Vailhev Shoal, off Grand Comoro Island, 220–440 m depth, 30 Aug. 1973.
Unknown location: HUMZ 102985, adult male 868 mm TL; HUMZ 124764, female 1013 mm TL.
Genetic material (specimens not retained). Indian Ocean : specimen #589 (GenBank accession ON167722 View Materials ), specimen #782 (GenBank accession ON167723 View Materials ), Myanmar waters of Andaman Sea, 2015; specimen J78 (GenBank accession ON167720 View Materials ), specimen J81 (GenBank accession ON167719 View Materials ), Kochi, India.
Eastern Atlantic: AZ-17 (GenBank accession JQ518946 View Materials ), male 860 mm TL, Funchal, Madeira, Portugal, 32°38′ N, 16°56′ W, 1 Jun. 2009; specimen #15, off central Angola, 11°30.95′ S, 13°21.24′ E, 22 Apr. 2010.
Western Atlantic: AM-1102-008-002-Csp (GenBank accession ON167711 View Materials ), off Pascagoula, Mississippi, northern Gulf of Mexico, USA, 29°2.78′ N, 88°32.27′ W, 26 Mar. 2011; OM-1009-005-002 (GenBank accession ON167712 View Materials ), OM-1009-005-005 (GenBank accession ON167713 View Materials ), off Pascagoula, Mississippi, northern Gulf of Mexico, USA, 29°8.74′ N, 88°16.6′ W, 15 Jan. 2011; OM-1009-XXX-002 (GenBank accession ON167714 View Materials ), off Pascagoula, Mississippi, northern Gulf of Mexico, USA.
Diagnosis. This species undergoes important morphoanatomic changes during ontogeny in many of the characters used for its identification. Comparison between pregnant females and the full-term embryos they contain, which are undoubtedly conspecific, provides the strongest evidence for such changes. The most notable changes are in coloration, morphology of the dermal denticles and morphology of the teeth. In addition, many morphological measurements show allometric trends, so their percentage value with respect to TL varies with body size. This pattern broadly follows that described for various species of sharks by Bass (1973). Thus, the description of characters which vary ontogenetically are provided first for full-term embryos and neonates and then for adults. Large juveniles and sub-adults present characters with intermediate values or morphologies. If nothing is indicated, the range of values includes the entire length range from newborn to adults even although there may be a trend in the values within this range
A medium-sized (112 cm maximum total length) species of Centrophorus with the following combination of characters: head long (length 20.4–25.1% TL) and moderately robust (width 10.4–14.3% TL); snout relatively short (horizontal preorbital length 4.8–7.2% TL), narrowly rounded in dorsal view; first dorsal fin relatively short (base length 10.9–16.0% TL; soft fin length 9.8–13.9% TL), moderately tall (height 5.2–7.7% TL); second dorsal fin slightly smaller than first (height of second dorsal fin 1.1–1.6 in that of first dorsal fin, soft fin length of second dorsal fin 1.2–1.6 in that of first dorsal fin); pectoral fins large (anterior margin length 10.7–13.4% TL), free rear tip moderately elongated in newborns, very elongate in larger individuals (sometimes with a damaged tip); dorsal and lateral surfaces medium greyish in newborns and small juveniles becoming pale brownish with a reddish tinge in large adults (some large adult males can have a uniform medium brown colour on entire body); inside of mouth almost black in newborns and small juveniles, whitish or pale grey in adults; dorsal fins newborns and small juveniles distinctly black apically with a white margin on free rear tips, the white margin lost in adults and dark markings less distinct but usually still present; central upper teeth erect, lateral teeth with oblique cusps and a marked notch; the number of erect (not asymmetric) teeth increases from a few (3–8) in newborns and small juveniles to most of them (>15) in adults and almost all (> 25 in large dark-brown males); lower teeth much larger than upper teeth, strongly oblique, blade-like with margin slightly serrated (only visible under magnification in small juveniles); lateral trunk denticles sessile (not raised on pedicels), changing from pointed in newborns and small juveniles to almost block-like in subadults and adults, not overlapping or elevated, margin scalloped; total vertebral centra 113–120 (mean 116); teeth 33–44 / 26–32.
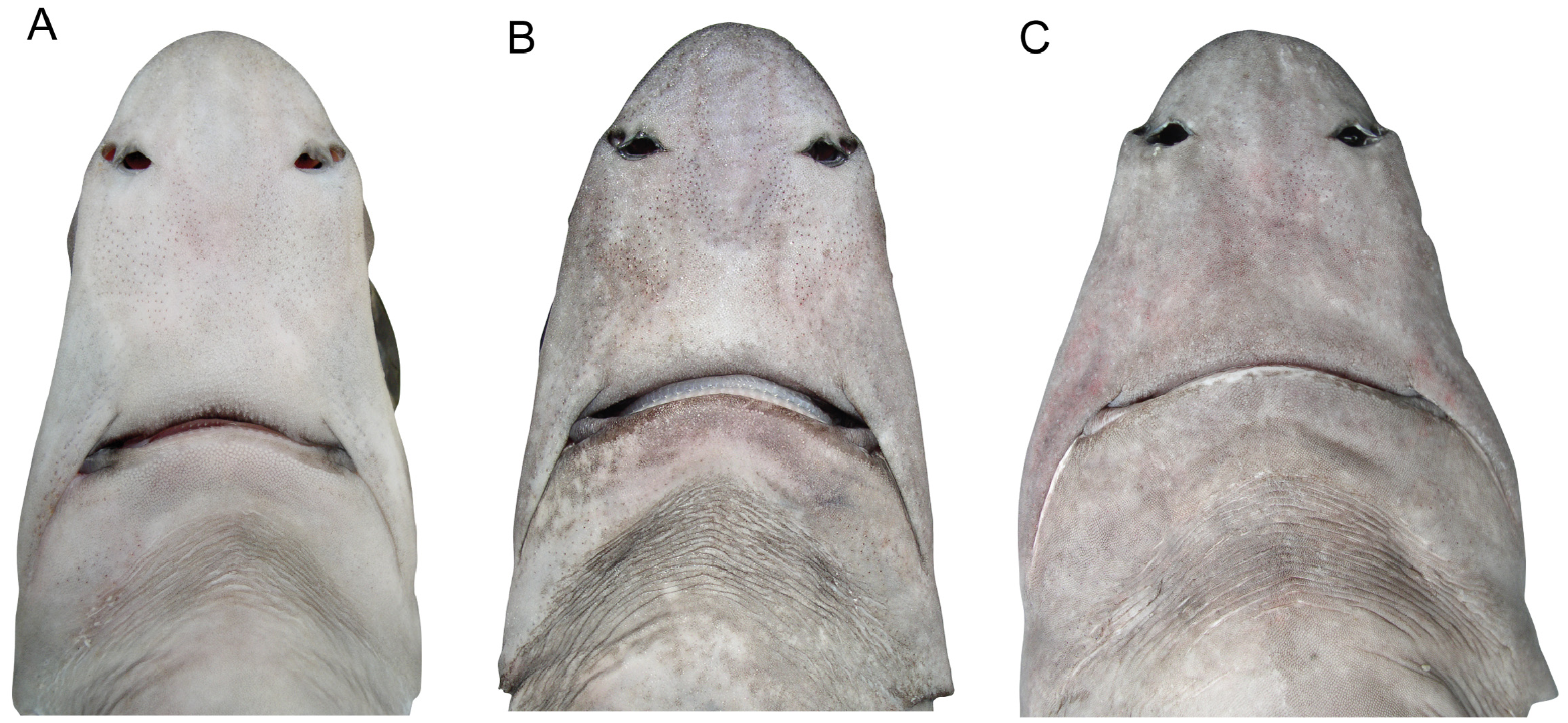
Description. Body fusiform, moderately elongate, nape moderately humped ( Figs 8 View FIGURE 8 , 9 View FIGURE 9 ); deepest near first dorsal-fin spine; head moderately elongate, length 22.5% TL in neotype (21.2–25.6% TL in 71 other specimens, from small juveniles to large adults); caudal peduncle moderately slender, pelvic-caudal space 13.9 (11.6–16.1)% TL. Head moderately robust, broad; depressed forward of spiracles, becoming somewhat semicircular in crosssection towards pectoral-fin origin; length 3.5 (3.0–3.8) in precaudal length; height 0.9 (0.7–1.0) times width. Snout relatively short, narrowly rounded in lateral view, apex bluntly pointed; lateral prenarial margin angular; narrowly rounded in dorsal view ( Fig. 10 View FIGURE 10 ); horizontal preorbital length 1.0 (0.8–1.2) times eye length, 0.6 (0.6– 0.9) times interorbital space; horizontal prenarial length 2.6 (2.2–2.8) times in preoral length.
Eye large, oval, length 4.2 (3.0–4.4) in head, 3.8 (2.4–4.7) times height; strongly notched posteriorly, notch not extending towards spiracle. Spiracle moderately-sized, semicircular; no lobe-like fold on posterior margin. Gill openings directed slightly anteroventrally from top to bottom; first four subequal in size, fifth longest, height of fifth slit 2.9 (2.3–4.1)% TL. Nostrils small, almost transverse; anterior nasal flap formed as a large subtriangular lobe with a somewhat rudimentary secondary lobe mesially; internarial space 2.5 (2.5–3.5) in preoral length, 2.5 (1.5–2.7) times nostril length. Mouth almost transverse ( Fig. 10 View FIGURE 10 ), upper jaw slightly concave, width 1.2 (0.9–1.4) in preoral length; upper labial furrows subequal to or slightly longer than lower furrows; prominent postoral groove, more than twice length of upper labial furrows, extending posterolaterally from angle of jaws.
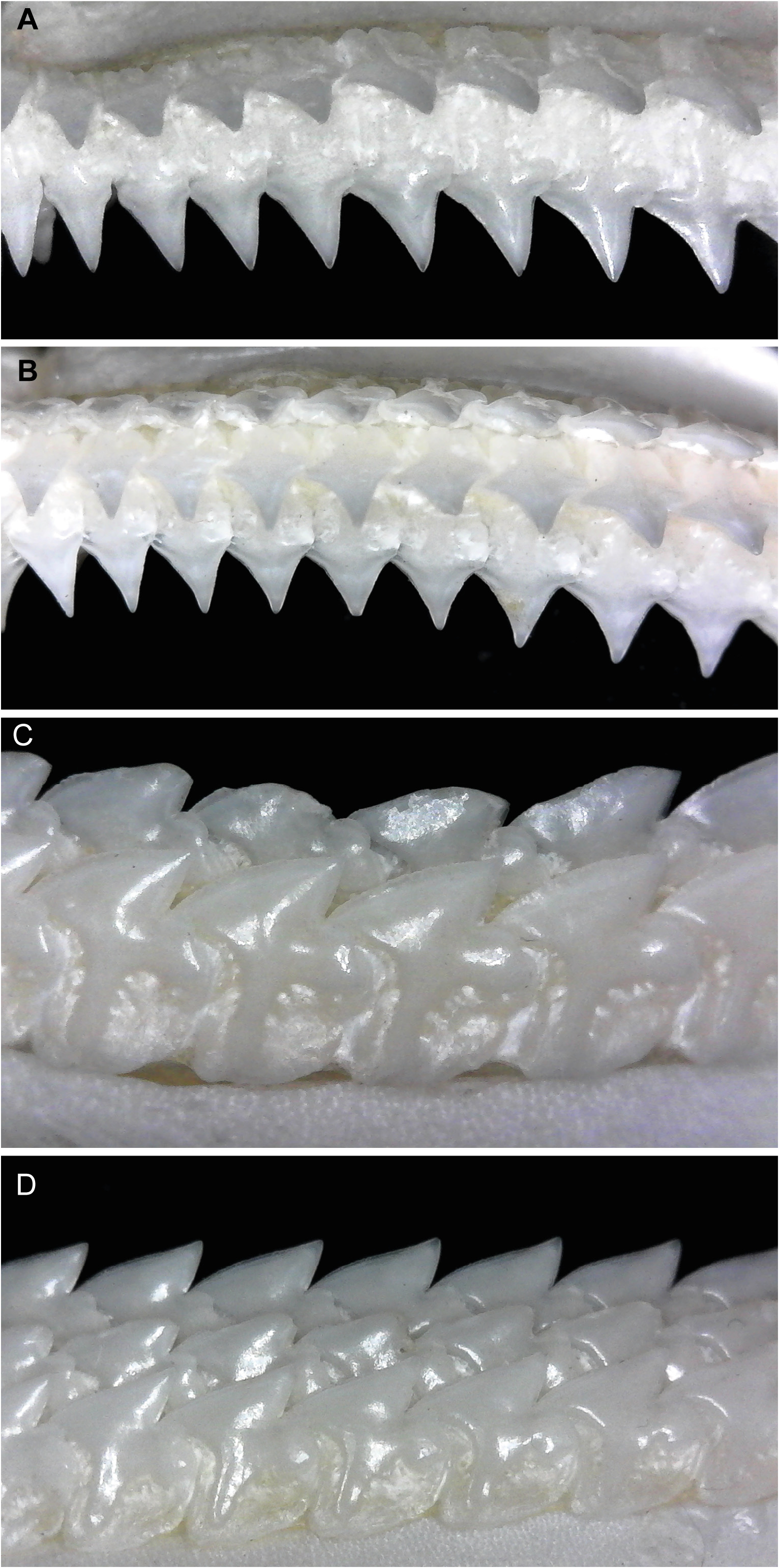
Dignathic heterodonty strongly evident; monognathic heterodonty noticeable in upper jaw and lesser so in the lower jaw; ontogenetic heterodonty non-existent in females, slight in males; sexual dimorphism evident in some adult males. Upper teeth mostly smooth in males, finely serrated in females ( Fig. 11A, C View FIGURE 11 ); no discernable symphyseal teeth present but with 3 to 6 very symmetrical, large and similarly-sized medial teeth with straight crowns and gradient merging into anterior files; upper tooth shape conical and somewhat narrow with peg-shaped roots and a distinct apron along basal face; anterior teeth with somewhat straight crowns and a very slight distal heel present and becoming more oblique antero-laterally; lateral teeth increasingly more oblique, deeply notched with a distinct distal bifurcation of basal and apical crown sections and a very distinct distal heel present; mesial crown portion of lateral teeth slightly concave with apical portion of crown slightly reflexed mesially; posterior files very strongly oblique and with heavily notched distal margins and straight mesial edges. Lower teeth with very well-developed serrations on mesial cutting edge, more so in adult females ( Fig. 11B, D View FIGURE 11 ); lower jaw with no discernable symphyseal or medial teeth; teeth compressed and interlocked with strongly oblique crowns forming an even cutting edge across lower jaw; anteroposterior files similar except for smaller size and lower crown height in posteriors; root asymmetrical with the distal edge slightly convex and the mesial edge concave and a very weakly developed apron at centre; crowns heavily notched distally with strong bifurcation of basal and apical crown sections and well-developed distal heels; mesial edge convex with apical portion directed distally; last file of teeth in posterior section usually with a very elongated distal root and basal crown section.
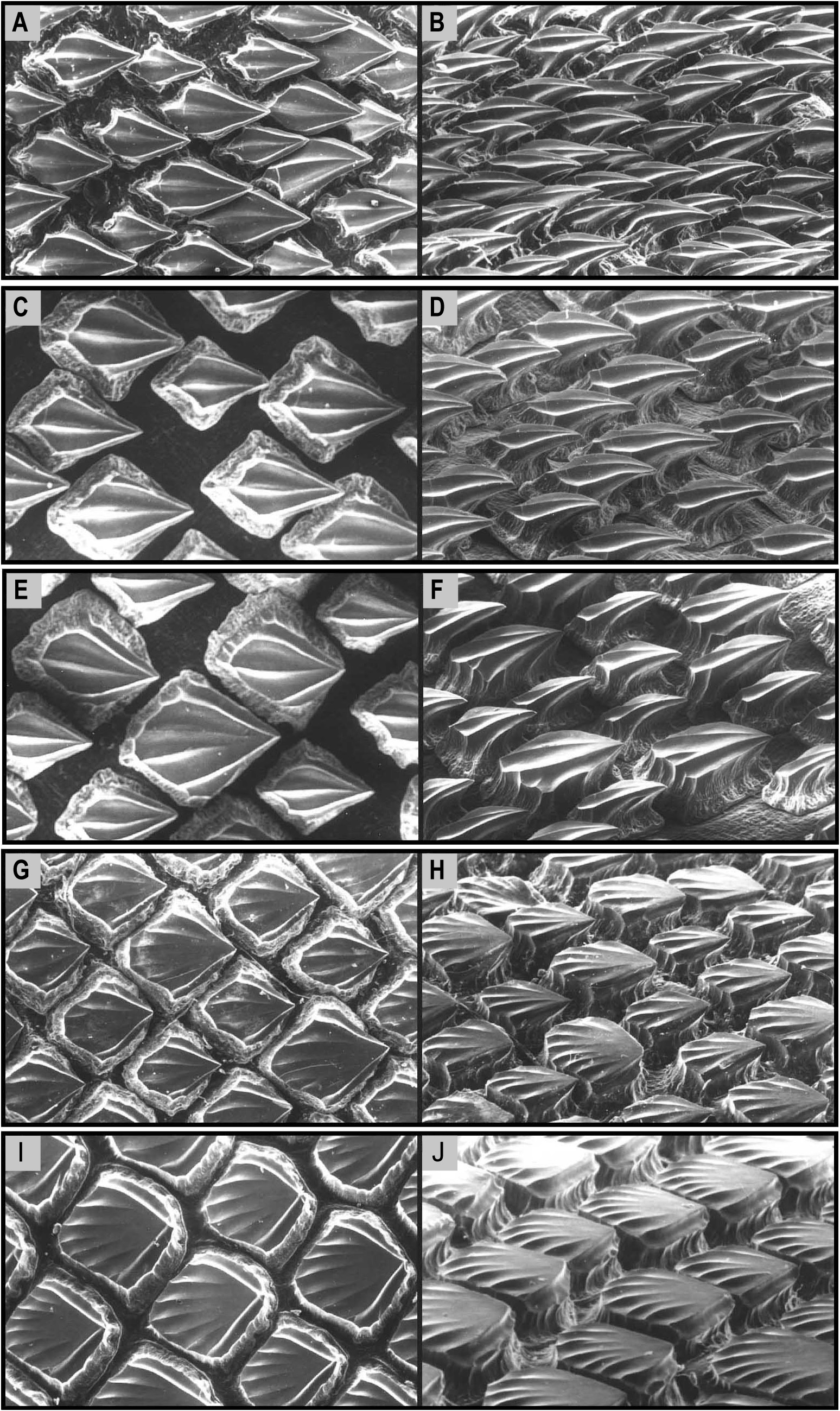
Dermal denticles on flank small, flat, pavement-like, not overlapping ( Fig. 4 View FIGURE 4 ); medial cusp relatively blunt; no lateral cusps; ridges present on anterior crown, not extending onto cusp. Denticles of newborns and small juveniles smaller, more pointed ( Fig. 4A, B View FIGURE 4 ); crowns with narrowly pointed medial cusp; a single central ridge distinct and extending to tip of cusp. Mid-sized juveniles with skin covered by small white flecks which under magnification are decalcified dermal denticles in a replacement phase.
First dorsal fin moderately small, raked, broadly rounded apically; anterior margin moderately convex; upper posterior margin straight to slightly convex, slanting strongly posteroventrally from top to bottom, moderately concave near free rear tip; free rear tip relatively thick basally, moderately long; inner margin of fin almost straight to weakly concave; insertion of base extremely well forward of pelvic-fin origin, posterior to or opposite free rear tip of pectoral fin; fin-spine origin above mid pectoral-fin inner margin (in newborns and small juveniles near the much shorter free tip apex); spine base broad, exposed anteriorly just below junction of spine and soft portion of fin; soft portion of fin connected about level of two thirds of total spine length; spine rapidly tapering distally when not damaged, anterior margin almost straight to weakly convex; exposed portion of spine sloping strongly posterodorsally from base to apex, usually shorter than exposed portion of second dorsal-fin spine; first dorsal soft-fin length 1.9 (1.5–2.3) times its height, 1.5 (1.2–1.6) times second dorsal soft-fin length; first dorsal-fin height 1.2 (1.1–1.6) times second dorsal-fin height; exposed first dorsal spine length 2.4 (2.0–4.6) in height of fin.
Second dorsal fin moderately small, slightly raked; anterior margin slightly convex, apex moderately to narrowly rounded; posterior margin weakly to moderately concave, sloping strongly posteroventrally from apex; free rear tip greatly elongated, inner margin length 0.9 (0.7–1.1) times fin height; second dorsal soft-fin length 1.6 (1.4–2.0) times its height; exposed spine length 2.3 (0.9–4.0) in height of fin; fin-spine origin above or just anterior to free rear tip of pelvic fin, exposed just below level of junction with spine and soft portion of fin; second dorsal spine moderately broad based, tapering distally, sharply pointed when undamaged; second dorsal-fin spine has a lateral expansion of enamel at apex that gives it an "arrowhead" appearance (not observed when the apex is eroded); interdorsal space 1.0 (0.9–1.3) in prepectoral length, 1.2 (1.2–1.7) in pre-first dorsal length; interdorsal groove weak.
Pectoral fin moderately large, anterior margin weakly convex; inner margin weakly convex anteriorly, almost straight posteriorly, anterior margin length 12.3 (10.7–13.4)% TL; apex moderately rounded to somewhat angular, lobe-like but not falcate; posterior margin almost straight from apex to free rear tip; inner margin length 12.3 (10.6–15.7)% TL; free rear tip only slightly elongate in newborns and small juveniles ( Fig. 12A View FIGURE 12 ), barely passing the origin of the spine of first dorsal fin, but greatly elongated in adults ( Fig. 12B View FIGURE 12 ), extending to posterior half of first dorsal-fin base; base very short, 2.1 (1.9–3.3) in anterior margin length.
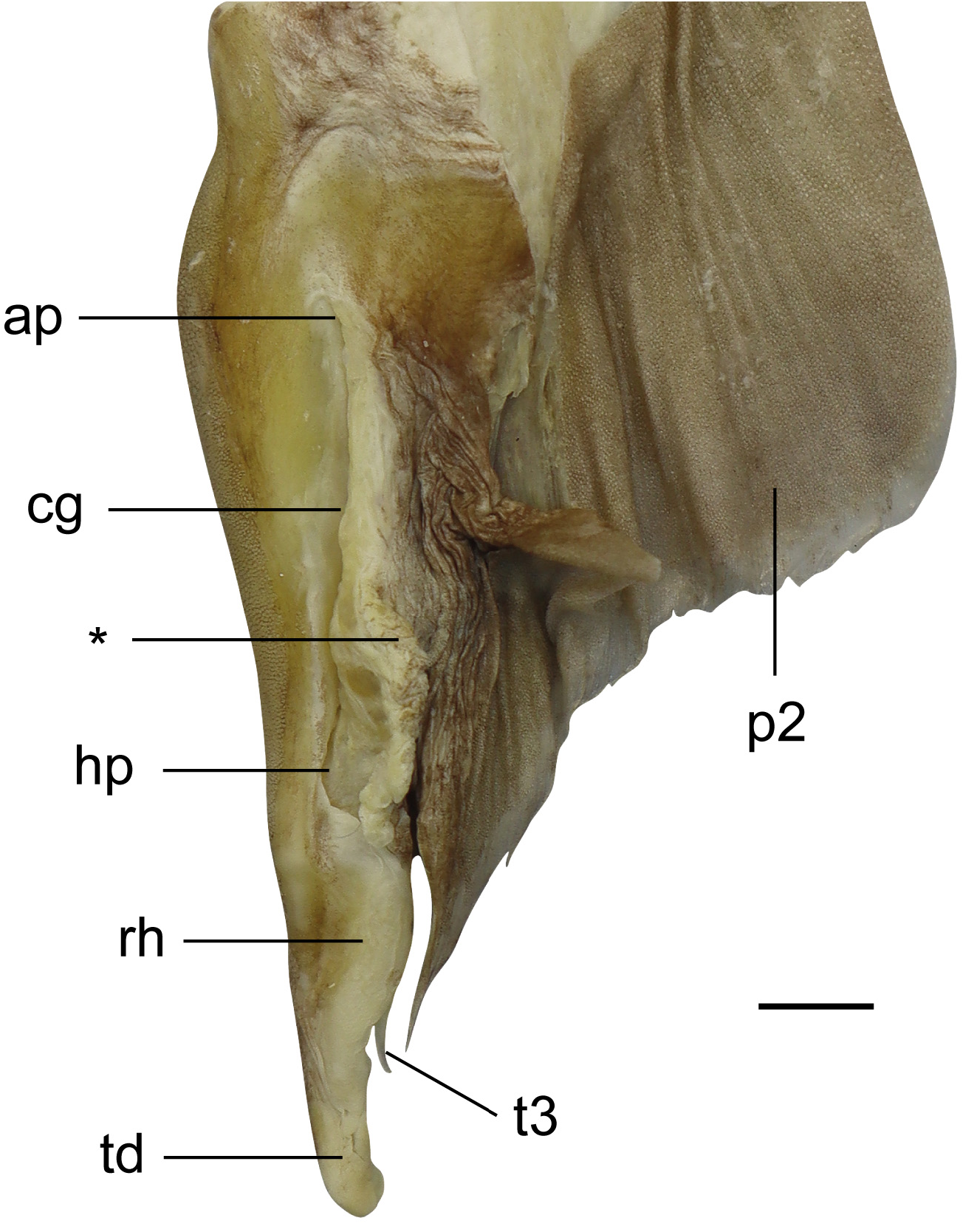
Pelvic fins moderately large, length 11.2 (9.8–12.8)% TL, anterior margin straight to weakly convex, posterior margin weakly concave to nearly straight, apex moderately rounded to bluntly angular, free rear tip acute to narrowly rounded. Claspers of adult males relatively short, outer length 2.6–4.0% TL, attached along inner margin of pelvic fin for most of their length, inner length 1.0–1.6 times pelvic-fin inner margin; area of attachment to pelvic fin markedly thickened; compressed dorsoventrally, only extended slightly beyond free rear tips of pelvic fin; clasper groove long, longitudinal and dorsomedially; apopyle and hypopyle not pronounced; rhipidion elongate, blade-like, mostly concealing the accessory terminal cartilage (spur); spur originating at about level of insertion of clasper and pelvic fin ( Fig. 13 View FIGURE 13 ).
Caudal peduncle moderately long, compressed, tapering slightly towards caudal fin; ventral groove weak; no lateral keels; pelvic–caudal space 2.3 (1.7–2.9) in pectoral–pelvic space, 1.6 (1.4–2.0) in prepectoral length; dorsal–caudal space 3.6 (2.3–3.5) in interdorsal length; precaudal pits absent. Caudal fin relatively long, postventral margin mostly moderately concave, terminal lobe moderately large, deep; apex of lower lobe narrowly to moderately rounded; dorsal caudal margin 1.1 (0.9–1.5) in head length; length of lower caudal lobe 1.8 (1.4–2.6) in upper lobe length.
Total vertebral centra 118 (113–120), monospondylous precaudal centra 56 (53–59), diplospondylous precaudal centra 30 (27–31), precaudal centra 86 (82–89) and diplospondylous caudal centra 32 (28–33). Tooth file count: upper jaw 16–22 (n = 25) + 17–22 (n = 27), 33–44 (n = 40); lower jaw 13–17 (n = 28) + 13–16 (n = 28), 26–33 (n = 37).
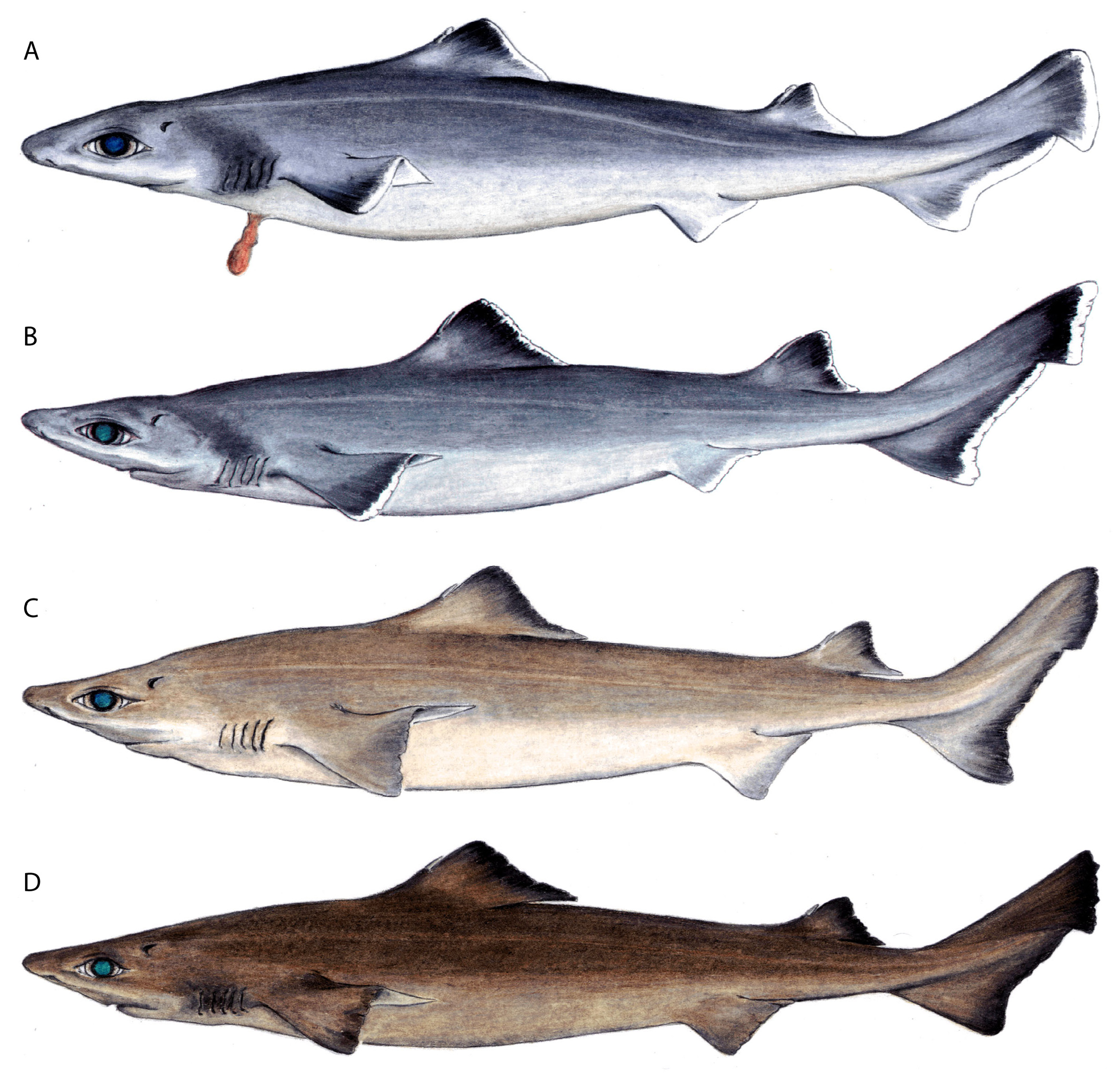
Coloration. Dorsal and lateral surfaces uniformly medium grey (newborns and small juveniles, Fig. 9 View FIGURE 9 , and some subadults and adults) to pale brown with a distinct pale reddish tinge (in larger adults, Fig. 8 View FIGURE 8 ) or uniform medium to dark brown (in some large adult males, Fig. 14 View FIGURE 14 ); ventral surfaces paler; waterline between dorsal and ventral colour shades very diffuse on lower sides, well demarcated on head, extending just below lower margin of eye and just below upper margin of gill slits, pectoral-fin origin pale; eye–spiracle space often with a distinct pale whitish blotch anteriorly, less distinct near spiracle. A diffuse white spot in the centre of the dorsal surface of the head in front of the eyes in both juveniles ( Fig. 9B View FIGURE 9 ) and adults. In subadults and adults, dorsal fins pale with a distinct, broad, diffuse-edged, dusky margin extending from about level of fin-spine apices to just posterior of the maximum concavity of the posterior margin; dorsal fin spines ivory coloured with a dark anterior longitudinal ridge covered with enamel; pectoral and pelvic fins darker distally, with narrow whitish posterior margins; caudal fin mostly greyish or brownish, postventral margin darker distally, terminal lobe usually darker than rest of fin. In newborns and small juveniles, a distinct dark greyish blotch over upper portion of gill slits, extending dorsally to level of upper margin of spiracle; dorsal fins blackish distally with a whitish free rear tip; pectoral fin upper surfaces dark grey with a distinct white posterior margin; pelvic fins pale with whitish posterior margin, blackish border on central edge of anterior margin; caudal fin with a broader white postventral margin, terminal lobe with very broad white margin and often with a darkish blotch extending to margin on lower third of terminal margin, preventral margin with a very narrow blackish border.
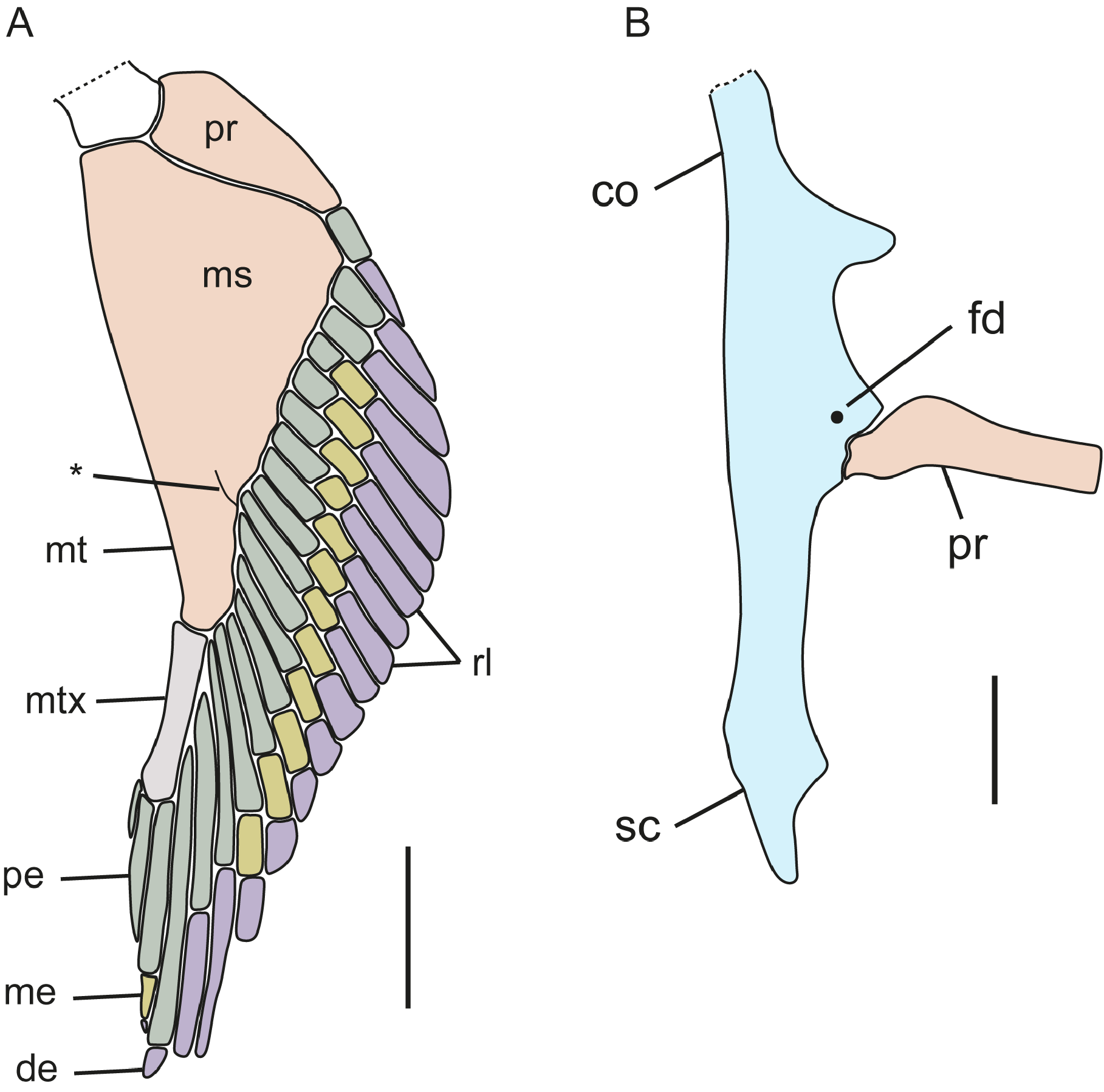
Skeletal morphology. Neurocranium ( Fig. 15 View FIGURE 15 ; based on CSIRO H 6310-04). Measurements of the neurocranium are presented in Table 2 View TABLE 2 . Neurocranium greatest width at level of postorbital processes (44.4–49.8% CL), narrower at interorbital region (29.0–33.4% CL). Rostrum elongate (precerebral fossa length 27.8–32.3% CL, its width 9.4–13.1% CL); lateral rostral appendages thick, short and hook-like; median rostral prominence small; rostral keel conspicuous and elongate, extending anterior to nasal capsules (its length 38.2–42.5% CL). Anterior fontanelle almost pear-shaped, located at base of rostrum and anterior to cerebrum; two short, broad protuberances anteriorly adjacent to nasal capsules. Nasal capsules almost spherical, moderately large, width across nasal capsules 40.8%–47.0% CL; inner anterior nasal margins with a small nasal protuberance; subnasal fossa moderately larger and suboval, located ventrally on each side of posterior rostral keel; a prominent, triangular, slightly curved process on anterolateral edge of each subnasal fossa.
Cranial roof strongly concave medially of interorbital region (forming a deep longitudinal sulcus on either side), moderately convex medially; a prominent lateral supraorbital crest present; preorbital canals large, tear-drop shaped, placed anteriorly to a series of foramina; canal for the ophtalmicus profundus small, rounded, located anterior to the preorbital canal and adjacent to ethmoidal canal; foramen of the epiphysial organ large, circular, located medially just posterior to anterior fontanelle; no supraethmoidal processes. Preorbital processes small, neurocranium broad between them. Postorbital processes prominent and triangular, not elongate, distance between processes 34.7–40.2% CL. Prominent ectethmoid process on each side of ethmoidal chamber; inconspicuous subethmoidal ridge, posterior to rostral keel, extending almost to subethmoidal region; subethmoidal region elongate and narrow, its width 5.4–7.9% CL.
Otic capsules relatively narrow; dorsally, two conspicuous anterior and two posterior semicircular canals; anterior canals with a strong ridge anteriorly; endolymphatic fossa oval and large, with two anterior endolymphatic foramina, slightly oblique, and two larger, posterior and vertical perilymphatic foramina; moderately strong otic crest located posteriorly to endolymphatic fossa; prominent sphenopterotic ridge at sides of otic capsules; opisthotic process at distal portion of sphenopterotic ridge small; laterally, otic wall delimited by a prominent lateral semicircular canal below the sphenopterotic ridge; width across hyomandibular facets 40.0–45.2% CL.
Orbital region narrow with concave preorbital wall, with orbitonasal canal at base; optic foramen (II) large, placed midventrally in interorbital wall; trochlear foramen (IV) small dorsal to optic foramen (II); eye-stalk located posteriorly, between the oculomotor foramen (III) and abducens foramen (VI); a broad foramen prooticum for trigeminal (V) and facial (VII) nerves positioned in the posterior edge of the interorbital wall, just anterior to the postorbital process; the foramen prooticum also opens posteriorly for the hyomandibular branch of the facial nerve (VII) at the base of hyomandibular facet; transbasal canal ventroposterior to anterior opening of foramen prooticum.
Basal plate flattened and large (length 33.4–40.2% CL), only slightly narrower anteriorly at basitrabecular process, broader posteriorly with its width 25.0–28.3% CL; basitrabecular processes conspicuous and elongate, slightly oblique to basal plate axis; basal angle width 15.3–17.4% CL; a small, shallow lateral prominence on each side at posterior of basitrabecular processes; two sets of cartilaginous processes on each side of the basal plate, first set small and below postorbital processes, second set prominent and below otic region, width across 2 nd posterior cartilaginous processes 30.2–34.4% CL; single foramen for carotid artery anteromedially located in basal plate; foramina for orbital artery with its ventral opening in the anterior base of the cartilaginous process and its lateral opening in the lateral otic wall.
Occipital region with two distinct, triangular occipital condyles distally, and a wide foramen magnum between them, its width 4.1–6.3% CL; vagus foramen (X) large, lateral to occipital condyles; narrow glossopharyngeal base located more laterally in the occipital region, with a narrow, oval foramen for glossopharyngeal nerve (IX).
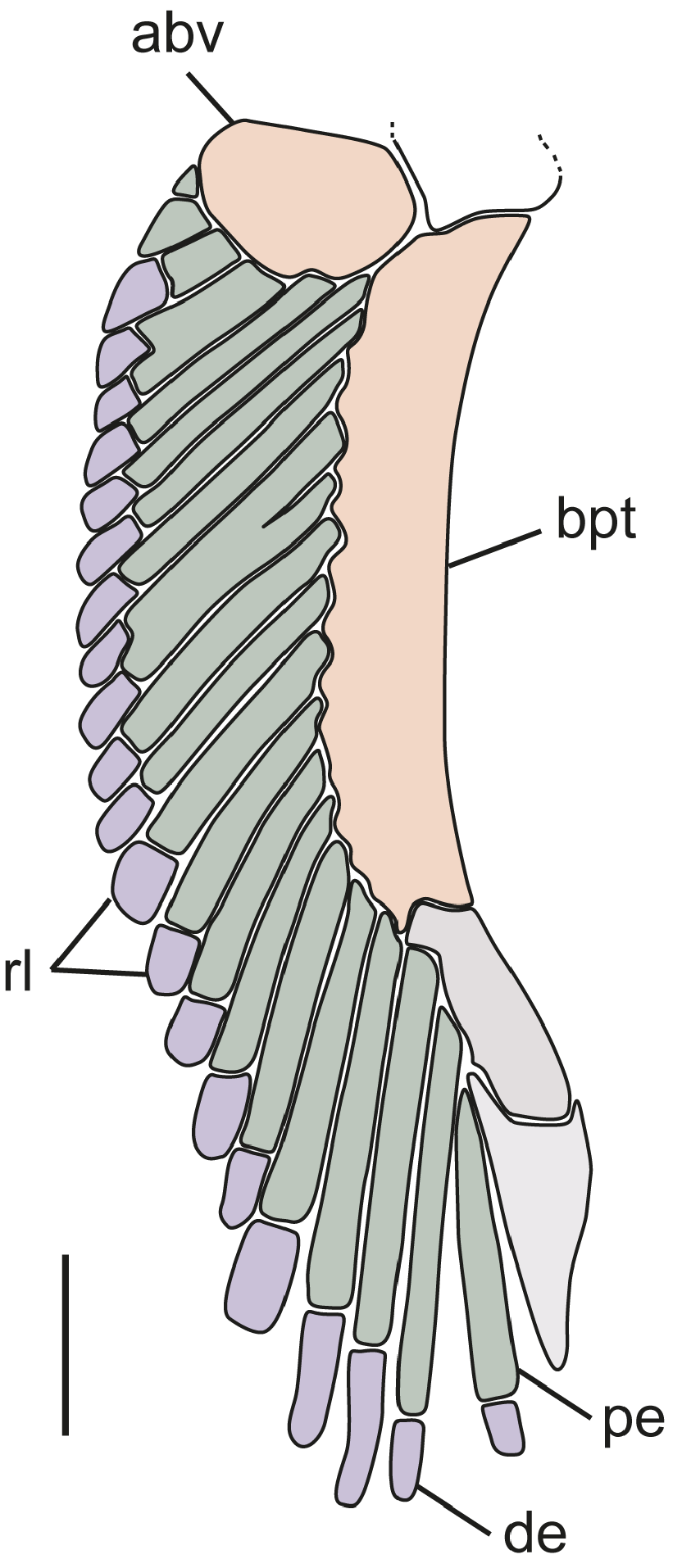
First dorsal fin ( Fig. 16 View FIGURE 16 ; based on CSIRO H 6503-04). Spine long and moderately wide basally, curving dorsoposteriorly. Dorsal fin with 6 basal plates bearing 8 proximal radials and 10 distal radials; first basal plate largest with 2 broad proximal radials anteriorly; a small triangular inter-basal plate at posterior of first basal plate bearing broad proximal radial which is divided distally with two separate distal radials; posterior 5 basal plates short and subequal in width, each bearing a single long proximal radial and a shorter distal radial; two posteriormost proximal radials curving posteriorly; penultimate proximal radial bearing two distal radials.
Guallart (1998) recorded first dorsal-fin radial counts of 5–11, mostly 7–9 (n = 154), and second dorsal-fin radial counts of 3–5, mostly 4 (n = 154).
Pectoral fin and girdle ( Fig. 17 View FIGURE 17 ; based on CSIRO H 6310-04). Coracoid bar convex anteriorly with a short, rounded projection on its medial portion ( Fig. 17A View FIGURE 17 ); posterior margin concave and with a faint medial notch; two conspicuous, posteriorly orientated triangular processes present on laterointernal coracoid bar; base of the scapula, on its junction with the coracoid bar, is expanded and provided with a rounded diazonal foramen located just anterior of the articular region; condyle for articulation of the pectoral basals is wide. Scapula forming a U-shaped scapulacoracoid cartilage; scapular process on each side relatively long and narrowly pointed. Pectoral fin with two pectoral basals: a wide and broadly subtriangular meso+mesopterygium and a shorter, narrow, subtriangular, distally enlarged propterygium; meso+mesopterygium mostly fused together but separation visible distally ( Fig. 17B View FIGURE 17 ). Pectoral radials: propterygium with one segmented radials consisting of a distal and proximal radial elements; mesopterygium with 9 segmented radials, two with only a distal and proximal radial element, remaining with distal, medial and proximal elements; metapterygium with 6 radials with 3 elements, 2 free radials, and a metapterygial axis with 3 radials. Guallart (1998) recorded pectoral-fin radial counts of 19–23, mostly 21 (n = 26).
Pelvic fin ( Fig. 18 View FIGURE 18 ; based on female CSIRO H 6503-04). Anterior pelvic fin basal element short and broad with 5 radials, distal one shortest; radials longer basally, becoming broader and shorter posteriorly; an additional rudimentary radial distal to other radials. Basipterygium elongate, slender, somewhat cylindrical, with 17 segmented radials; proximal elements of fourth and fifth radials fused distally; terminal axis with a broad, moderate-sized, cylindrical proximal cartilage element and an elongate triangular, flattened distal element. Pelvic radials thin and long, cylindrical, and segmented into a long proximal element and much shorter distal element; 22 total pelvic radials. Guallart (1998) recorded pelvic-fin radial counts of 18–20 in males (n = 9) and 22–24 in females (n = 7).
Claspers ( Fig. 19 View FIGURE 19 ; based on right clasper of CSIRO H 6309-02). Axial cartilage slender, long, curved along its length; dorsal marginal cartilage slim, located dorsolaterally to axial cartilage; dorsal terminal cartilage elongate, reaching almost to distal end of clasper, connected proximally to dorsal marginal cartilage and axial cartilage; dorsal terminal 2 cartilage wider, flattened, also elongate, with concave lateral margin, attached medially to dorsal terminal cartilage, and proximally to dorsal marginal cartilage; ventral marginal cartilage flat and quadrangular, emerging as a folded plate at insertion of accessory terminal cartilage; ventral terminal cartilage large, elongate, slender, curved distally with a bluntly rounded distal tip; accessory terminal 3 cartilage (or spur) slim and elongate with an evident dorsal groove, distally pointed, partially attached to ventral margin and ventral terminal cartilages. Additional information on clasper morphology is available in Guallart (1998).
Size. The largest specimen examined in this study was a 1059 mm TL individual from the Canary Islands. In the Mediterranean, this species has been recorded to 1125 mm TL by Guallart (1998) and in Australia it has been reported to 1110 mm TL by Graham & Daley (2001). Although Capapé (1985) recorded a maximum size of 1280 mm TL for this species in the Mediterranean Sea, it did not appear in any of the graphs provided in that publication and we consider is a doubtful maximum size for this species. Minimum size at maturity recorded for C. uyato appears to be relatively consistent across its range: Capapé (1985) and Megalofonou & Chatzispyrou (2006) reported a size at maturity of 745–800 mm TL for males and 850–940 mm TL for females in the Mediterranean; likewise, Guallart (1998) reported size range of maturation of 790–850 mm TL for males (L 50 = 799 mm TL) and 893–994 mm TL for females (L 50 = 935 mm TL) in the Mediterranean; size at maturity off southern Australia is 790 mm TL for males and 960 mm TL for females. Size at birth 330–461 mm TL in the Mediterranean ( Capapé, 1985; Guallart & Vicent, 2001) and 380–450 mm TL in southern Australia.
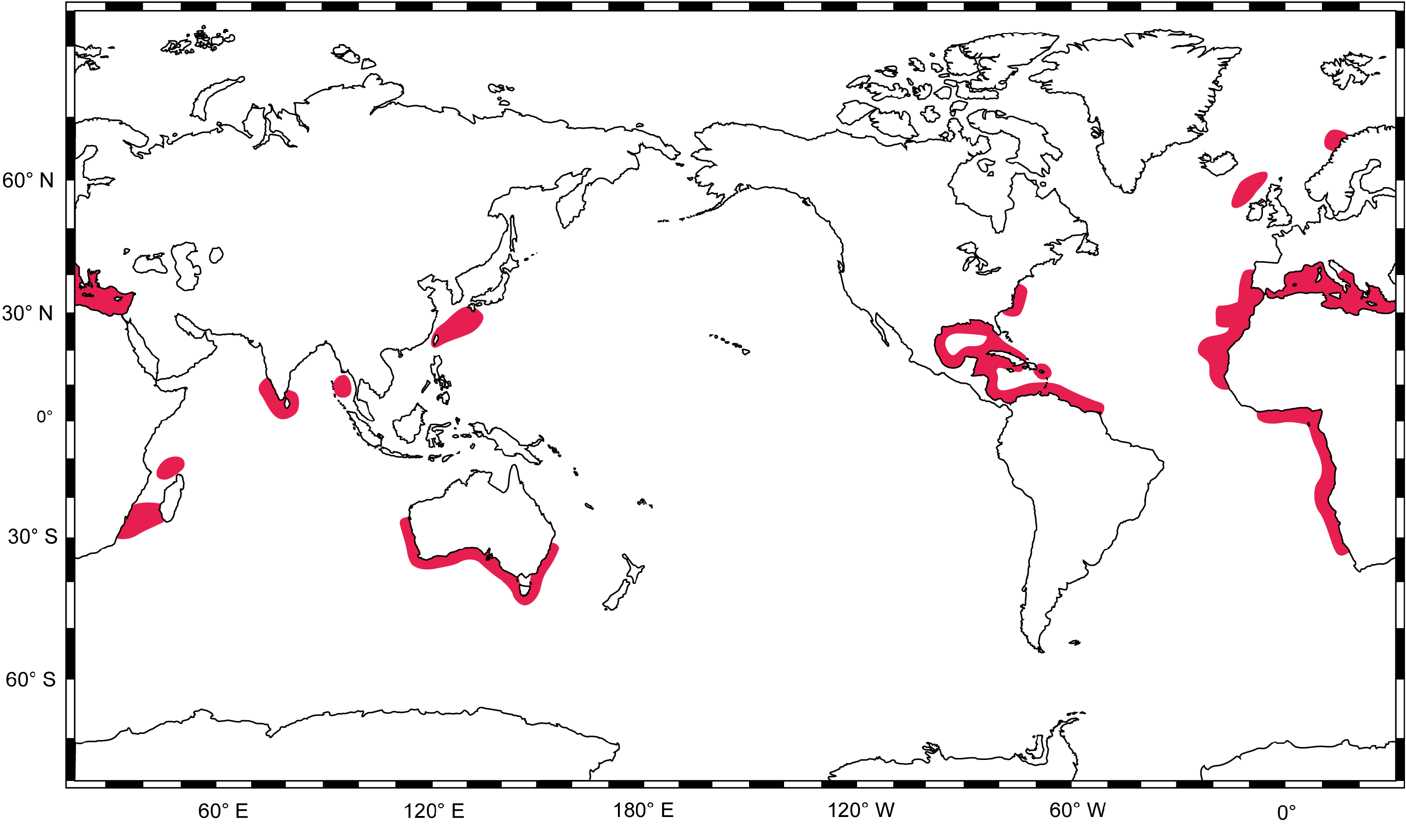
Distribution. Known from scattered localities around the world ( Fig. 20 View FIGURE 20 ): Indo-Pacific: southern Australia (Forster in New South Wales to Freycinet Estuary in Western Australia, including Tasmania) ( White et al., 2008); Taiwan ( Compagno, 1984a; Serena, 2005); East China Sea, Japan ( Bessho, 2006); Okinawa, Japan (G. Naylor, unpubl. data - GN10528); Andaman Sea, Myanmar (GenBank accession ON167722 View Materials ); Sri Lanka ( Morón et al., 1998; Fernando et al., 2019); Kochi, India (GenBank accession ON167719 View Materials and ON167720 View Materials ); Kollam, India ( Bineesh et al., 2016); Comoro Islands ( Compagno, 1988a); Mozambique Channel, Madagascar (G. Naylor, unpubl. data - GN5684); southern Mozambique ( Bass et al., 1976); and Natal, South Africa ( Springer, 1990). Eastern Atlantic: Hondeklip Bay, South Africa ( Compagno et al., 1991); Namibia ( Compagno, 1984a; Bessho, 2006); Angola ( Poll, 1951; GenBank accession ON167704 View Materials ); Cape Verde ( Reiner, 1996; GenBank accession ON167721 View Materials ); Angola to Ivory Coast, Senegal to Gibraltar ( Compagno, 1984a); Senegal and Gambia ( Rochebrune, 1883); Western Sahara ( Belloc, 1934); Madeira, Portugal ( Maul, 1955; Biscoito et al., 2018); Canary Islands ( Maurin & Bonnet, 1970; Pajuelo et al., 2010); Atlantic slope off Morocco ( Belloc, 1934); continental coast of Portugal ( Costa et al., 2012); Rockall Trough, NW of Ireland ( Clarke, 2000); west of Britain ( Bridger, 1978); northern Norway ( Wienerroither et al., 2015). Mediterranean Sea (where it is the only species of Centrophorus present): Spain ( Guallart, 1998; Guijarro et al., 2012; Barría et al., 2015a, b); France ( Rancurel, 1983; Capapé et al., 2000; Barría et al., 2015a, b); Italy ( Tortonese, 1956; Parenti, 2019); Croatia ( Gajić, 2019); Montenegro ( Gajić, 2019); Albania ( Ungaro et al., 1999; Gajić, 2019); Malta ( Lanfranco, 1996; Schembri et al., 2003); Greece ( Megalofonou & Chatzispyrou, 2006); Turkey ( Bilecenoglu et al., 2002; Meriç et al., 2007); Syria ( Gruvel, 1931; Ali & Saad, 2003); Lebanon ( Colloca & Lelli, 2012; Lteif, 2015; Bariche & Fricke, 2020); Israel ( Golani & Pisanty, 2000; Goren & Galil, 2015); Cyprus ( Hadjichristophorou, 2006; Kousteni et al., 2021); Eypgt ( Farrag et al., 2016); Libya ( Zupanovic & El-Buni, 1982; Serét, 2005); Tunisia ( Farrugio & Soldo, 2014); Algeria ( Duméril, 1865; Boutan, 1926; Maurin, 1962); Morocco ( Maurin, 1962; 1968). Western Atlantic: Suriname and French Guiana ( Uyeno et al., 1983); Venezuela ( Cervigón & Alcalá, 1999; Ehemann et al., 2019); Caribbean Colombia ( Hernández-Hamón & Núñez, 1998); Mexico ( Applegate et al., 1993; Bonfil, 1997); Cuba ( Guitart, 1979); Louisiana to Florida, Gulf of Mexico, USA ( Perry et al., 1995; Veríssimo et al., 2014; Hipes, 2015; Driggers et al., 2017); Florida Straits to Dry Tortugas and South Carolina, USA ( Davenport et al., 2011); North Carolina and Virginia, USA ( Kiraly et al., 2003); and US Virgin Islands ( Kiraly et al., 2003).
Recorded from depths of 208–701 m, usually greater than 400 m, off Australia ( White et al., 2008). In the Mediterranean Sea, abundant commercial fishing catches have been reported by Boutan (1926) at 150–500 m in Algeria; Rancurel (1983) at 150–600 m in Corsica; Guallart (1998) at 150–650 m depth in the Balearic Sea. Guallart (1998) reported that catches of up to 900 subadult and adult specimens could be landed in a single day ( Fig. 21 View FIGURE 21 ). Muñoz-Chapuli (1984) reported the uncommon catch of a juvenile in the continental shelf at “<100 m depth” in NE Atlantic. Compagno et al. (1989) reported a depth range of 274–480 m off southern Africa. Maximum depths reported for this species is 1490 m ( Gilat & Gelman, 1984) and in some other cases about 1400 m (e.g. FAO, 2018; Psomadakis et al., 2019), but rarely deeper than 600 m (Baino et al., 2001).
Intraspecific variation. The MDS plot for C. uyato coded by size classes shows a trend from smaller size classes to the left of plot to larger size classes to the right of the plot ( Fig. 22 View FIGURE 22 ). The samples for the larger size classes show considerable overlap, but those for size class 1 (<500 mm TL) and 2 (500–699 mm TL) are grouped away from the larger size class samples. ANOSIM showed size classes were significantly different overall (P <0.01), albeit with relatively low support (R 2 = 0.466). Pairwise comparisons were only significantly different (P <0.01) between size class 1 (<500 mm TL) and all other size classes, and between size class 2 (500–699 mm TL) and size classes 4 (800–899 mm TL) and 5 (> 900 mm TL). The measurements shown by SIMPER to be the most responsible for differences between size class 1 and the other size classes are (in order of importance): pectoral–pelvic space (e.g. 24.4–29.3 vs. 28.8–33.1% TL in size class 4), dorsal caudal margin (e.g. 20.3–22.8 vs. 17.2–21.1% TL in size class 5), pectoral-fin inner margin length (10.6–12.9 vs. 11.5–15.7% TL in size class 4), and head width (e.g. 10.4–12.9 vs. 12.5–14.2% TL in size class 3). The measurements shown by SIMPER to be most responsible for differences between size class 2 and the size classes 4 and 5 are (in order of importance): pectoral–pelvic space (27.4–30.6 vs. 27.6–36.1% TL), pelvic-fin height (4.5–5.5 vs. 5.3–7.4% TL) and dorsal caudal margin (19.8–22.5 vs. 17.2–21.1% TL). It is important to note that the characters found by SIMPER to distinguish between the size classes mostly overlap considerably.
The most notable differences between juveniles and larger specimens of C. uyato are coloration and dermal denticle morphology. Juveniles have very distinct markings on the dorsal and caudal fins, which adults lack, and body coloration changes from medium grey in juveniles to brown in adults ( Fig. 23 View FIGURE 23 ). The lateral trunk denticles of juveniles have far more pointed cusps than larger specimens, which have more pavement-like, flat denticles ( Fig. 4 View FIGURE 4 ).
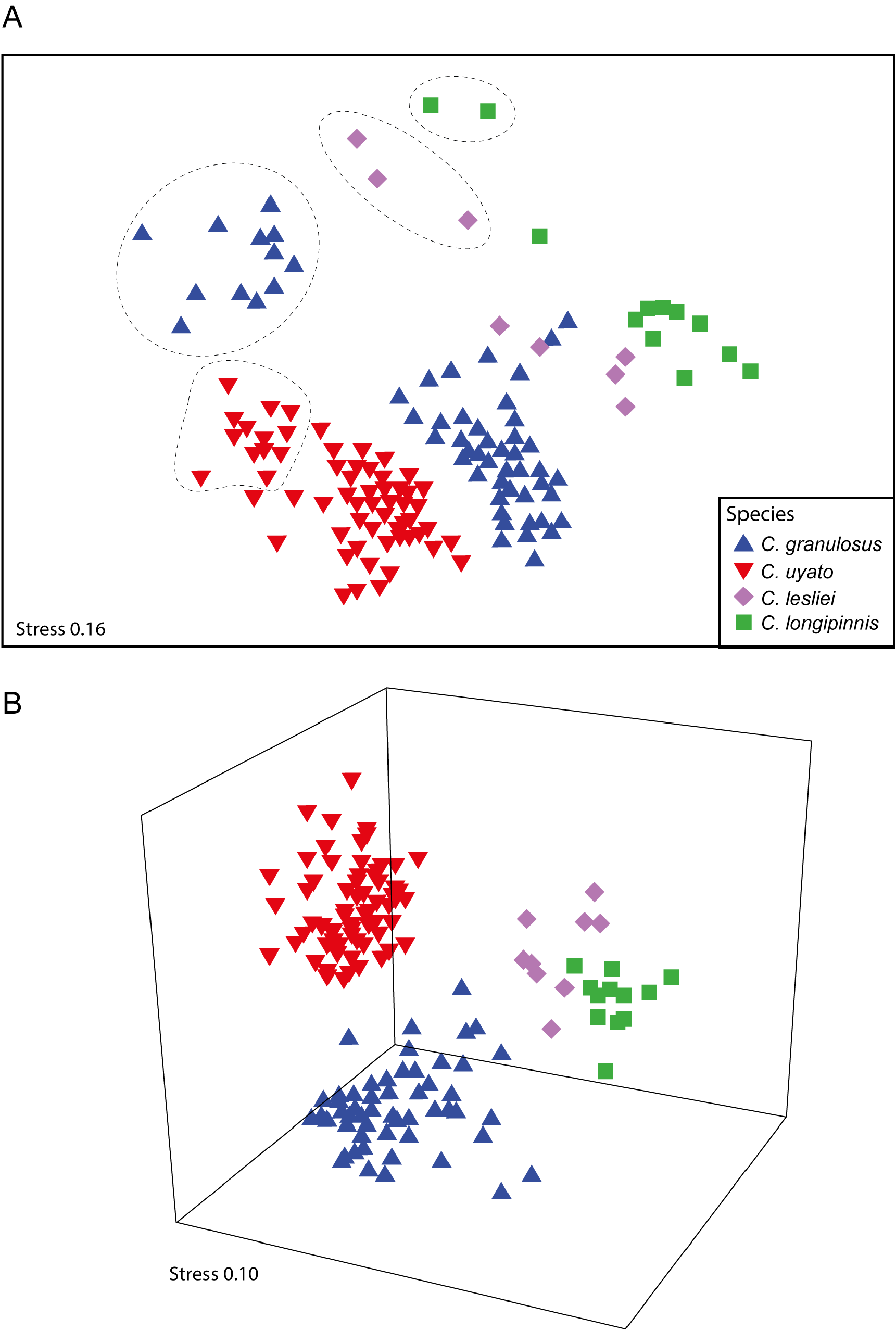
The ontogenetic differences in morphology within members of this genus are well illustrated in Fig. 24A View FIGURE 24 , which incorporates the four species so far investigated in this revision series (i.e. C. granulosus , C. lesliei , C. longipinnis and now C. uyato ). Small individuals of C. granulosus (<700 mm TL) were found to differ significantly from individuals> 820 mm TL in having a longer head, larger eyes, paired fins closer together, a taller second dorsal-fin spine and different denticles morphology ( White et al., 2013). Small individuals of C. uyato also differ in denticle morphology and in having the paired fins closer together and a slightly taller second dorsalfin spine compared to larger individuals. However, there were no difference in measurements associated with a longer head (i.e. preoral length, preorbital length, prespiracular and prenarial length) or eye size. Similarly, small individuals of C. lesliei and C. longipinnis (<524 mm TL) differed from the larger individuals in having a longer head, shorter pectoral-pelvic space and eye length ( White et al., 2017). The smaller size classes of these two species also differed from larger individuals in having a longer caudal fin and pectoral-fin inner margin, which was also the case for C. uyato . Thus, ontogenetic shift in morphology in this genus appears to be driven by a relatively small number of features. Examination of this ontogenetic variation should be included in future studies of not only this genus, but all groups where possible. In general, variation within species of sharks is poorly understood despite adequate specimens often available for investigation.
Comparison between species. Centrophorus uyato differs from the two large species of Centrophorus , i.e. C. granulosus and C. squamosus in the following key characters: denticles of adults flat, pavement-like (vs. raised on pedicels or tear-drop shaped) in subadults and adults; pectoral-fin free rear tip in subadults and adults elongate (vs. not or only slightly produced); body medium brown to greyish above, paler below (vs. mostly uniform dark brown, except in juvenile C. granulosus ). It differs from C. moluccensis in having a taller second dorsal fin (its height 4.0–6.1 vs. 2.9–3.9% TL, 1.1–1.6 vs. 1.6–2.2 times, respectively, in first dorsal-fin height). Centrophorus uyato can be readily distinguished from C. lesliei and C. longipinnis in having a much shorter first dorsal fin (first dorsal soft fin length 9.8–13.9 vs. 16.0–20.2% TL, 1.2–1.6 vs. 1.7–2.3 times in second dorsal soft fin length).
Centrophorus uyato is superficially similar to members of the long-snouted species complex that consists of C. harrissoni , C. isodon , C. seychellorum , C. westraliensis and possibly C. tesselatus . A revision of the long-snouted complex is currently in progress and will be presented as another part of this series of revision papers for the genus Centrophorus . Centrophorus uyato is sympatric with C. harrissoni and C. westraliensis in Australian waters and their similarity has led to these species being commonly mis-identified. It differs from these two species in having a shorter snout (preorbital length 4.8–7.2 vs. 7.5–8.6% TL, prenarial length 3.3–4.7 vs. 5.9–5.8% TL); shorter preoral length (in adults, 8.2–11.2 vs. 10.9–12.4% TL); dorsal fins of subadults and adults with a blackish apical marking (usually obvious when fresh) vs. without a dark apical marking but with narrow white posterior margin (sometimes indistinct in large adults); dorsal fins of juveniles with distinct, broad blackish apical markings and pale free rear tips vs. a black anterior blotch and white posterior blotch.
It is difficult to provide accurate data to compare Centrophorus uyato with C. isodon , C. seychellorum and C. tesselatus as they are, at present, poorly defined. The molecular data presented in White et al. (2017) shows C. harrissoni forming a group sister to samples consisting of specimens identified as C. isodon ( Indonesia) , C. westraliensis (Western Australia) and C. tesselatus (Gulf of Mexico). However, another group of samples containing C. isodon ( Philippines) and C. tesselatus ( Japan) is also present.
The dental morphology of Centrophorus species often passes through various stages of ontogenetic development, but this can be inconsistent at times often rendering dentition a difficult diagnostic tool for some species. In addition, sexual dimorphism in Centrophorus uyato is not always consistent, particularly in males. Immature males often have oblique, weakly developed cusps which may become very erect and conical in shape with maturity. However, this is sometimes only weakly developed and immature males quite often will have predeveloped teeth with small, very erect cusps. Adding to the confusion, juvenile males at times may have very small, erect lower tooth crowns with somewhat concave mesial margins, mesially reflexed cusp tips and heavy serrations. In some instances, this has led to the misidentification of immature specimens of larger species of Centrophorus .
Regarding the comparison of the dental morphology of Centrophorus uyato with other small species of Centrophorus , for the most part, female Centrophorus uyato will tend to have much longer, erect and better developed upper anterolateral tooth crowns than other species. For example, female C. moluccensis , C. isodon and C. harrissoni tend to have very oblique crowns, more reminiscent of Squalus . Additionally, the 3–6 straight and symmetrical upper medial teeth, as noted in the description, are seldom seen in other species of Centrophorus aside for C. granulosus and sometimes C. squamosus . A detailed comparison of dentition for species of Centrophorus is currently in preparation and is beyond the scope if this publication.
The MDS plot based on morphometric measurements of the four species of Centrophorus for which this series of revisions has included to date (i.e. C. granulosus , C. lesliei , C. longipinnis and now C. uyato ) separated the species well ( Fig. 24 View FIGURE 24 ). Although the two-dimensional plot ( Fig. 24A View FIGURE 24 ) shows two C. granulosus samples overlapping with C. lesliei samples, this is an artifact of the two-dimensional plot and the three-dimensional plot ( Fig. 24B View FIGURE 24 ) shows clearer separation. ANOSIM showed morphological measurements were significantly different between species (P <0.01; R 2 = 0.690), and in all pairwise comparisons (P <0.01; R 2 = 0.547 –0.994), except for C. lesliei vs. C. longipinnis which had only a weak significant difference (P <0.5; 0.254). The measurements shown by SIMPER to be most responsible for differences between C. uyato and both C. lesliei and C. longipinnis are not surprisingly related to length of the first dorsal fin: first dorsal-fin base (10.9–16.0 vs. 16.8–23.3% TL), first dorsal soft fin length (9.8–13.9 vs. 16.0–20.2% TL) and first dorsal-fin length (16.8–21.9 vs. 23.1–29.4% TL). The measurements shown by SIMPER to be most responsible for differences between C. uyato and C. granulosus are: second dorsal soft fin length (7.3–9.0 vs. 9.3–11.6% TL); pectoral–pelvic space (24.4–36.1 vs. 27.4–39.2% TL); interdorsal space (18.2–24.3 vs. 14.6–21.1% TL) and first dorsal soft fin length (9.8–13.9 vs. 11.0–16.6% TL). Although the range in measurements for each of these characters overlap in most cases, the high level of intraspecific variation in both species, particularly in relation to ontogenetic changes in morphology, is the likely the main cause.
For each species, the smallest size class is located to the upper left of the remaining size classes. When only size class 1 is compared between the four species, ANOSIM showed a more significant difference between species (P <0.01; R 2 = 0.962) that when all size classes were included. Likewise, when all other size classes (i.e. excluding size class 1) where compared between species, ANOSIM also showed a more significant difference (P <0.01; R 2 = 0.881) than when all size classes included. Thus, ontogenetic differences in morphology are an important consideration when comparing species of Centrophorus (see previous section). The characters shown by SIMPER to contribute the most to the differences between species for both of the above analyses separated by size class are displayed in Table 3 View TABLE 3 . There is little variation in the characters SIMPER found that best differentiated between species with the exception between C. uyato and C. granulosus where different characters best distinguished between size class 1 individuals and between large individuals (excluding size class 1).
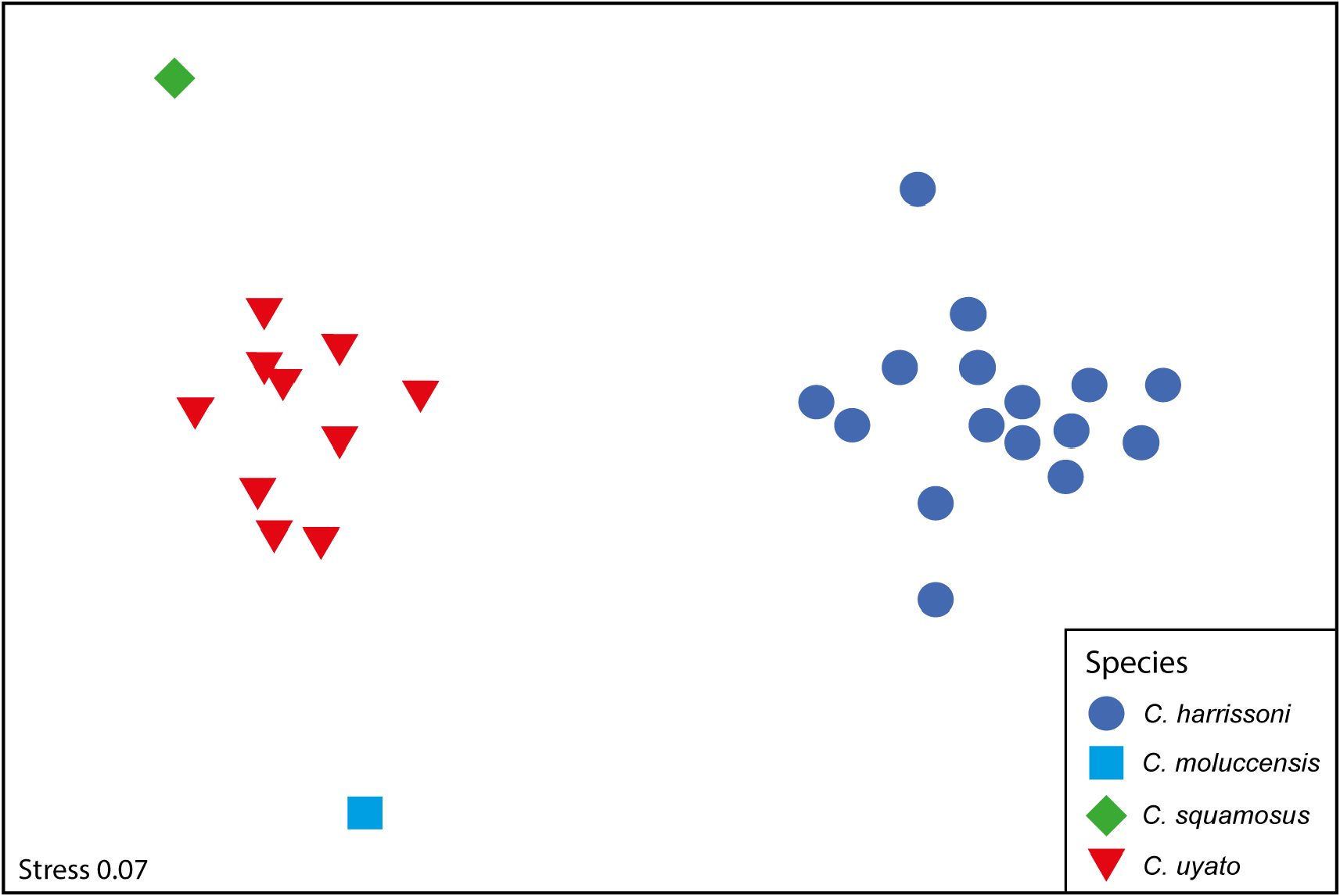
The morphology of the neurocranium is also a useful character for separating Centrophorus species. While not a useful field character, neurocranium morphology in Centrophorus should be investigated further. For the four species examined, the MDS plot based on proportional neurocranium measurements separated the species well ( Fig. 25 View FIGURE 25 ). The samples for C. harrissoni grouped together on the right side of the plot, well away from the other three species. Centrophorus uyato samples grouped tightly together in the centre of the left side of the plot, with the single samples of C. moluccensis and C. squamosus located above and below the C. uyato samples, respectively. ANOSIM showed neurocranial measurements were significantly different between species (P <0.01; R 2 = 0.994), and in all pairwise comparisons. The measurements shown by SIMPER to be most responsible for differences between C. uyato and C. harrissoni are (in order of importance): nasobasal length (61.6–65.2 vs. 48.0–58.2% CL), rostral keel length (38.2–42.5 vs. 46.7–54.9% CL), width across postorbital processes (44.4–49.8 vs. 36.1–44.0% CL), and precerebral fossa length (27.8–32.3 vs. 33.6–39.7% CL). In addition, they also differ in interorbital width (29.0–33.4 vs. 25.3–28.0% CL). Muñoz-Chapuli & Ramos (1989) compared the neurocranium of C. granulosus (as C. niaukang ), C. uyato (as C. granulosus ) and C. lesliei (as C. lusitanicus ). The main characters used to separate C. uyato from the other two species was width of the precerebral fossa and height of neurocranium. Based on seven specimens, Muñoz-Chapuli & Ramos (1989) recorded a mean precerebral fossa width for C. granulosus of 7.70% CL and a maximum sagittal height of 24.96% CL, vs. a mean of 12.19% CL and 28.97% CL in 18 specimens of C. uyato , respectively.
The measurements shown by SIMPER to be most responsible for differences between C. uyato and C. moluccensis are: postorbital width (44.4–49.8 vs. 38.2% CL), interorbital width (29.0–33.4 vs. 25.3% CL), and width across nasal capsule (40.8–47.0 vs. 38.9% CL). SIMPER found the main differences between C. uyato and C. squamosus are: width of basal angle (15.3–17.4 vs. 22.6% CL), postorbital to prootic processes (19.9–23.1 vs. 26.4% CL), and interorbital width (29.0–33.4 vs. 27.0% CL). Note for both C. moluccensis and C. squamosus , only a single neurocranium was measured. Thus, the differences noted above need to be investigated further when additional crania become available for these species.
Comparative material
Comparative material examined in this study is listed in White et al. (2013, 2017) in the material examined and comparative material sections. Additional comparative material is listed below:
Centrophorus harrissoni . CSIRO H 6307–02 (skeletal parts), female 1066 mm TL, CSIRO H 6307–03 (skeletal parts), juvenile male 557 mm TL, CSIRO H 6307–04 (skeletal parts), female 1025 mm TL, CSIRO H 6307–05 (skeletal parts), juvenile male 565 mm TL, CSIRO H 6307–06 (skeletal parts), female 933 mm TL, east of Flinders Island, Tasmania, ~ 40° S, ~ 149° E, 350–430 m, 12 Jul. 2004; CSIRO H 6308–02 (skeletal parts), female 1039 mm TL, CSIRO H 6308–03 (skeletal parts), female 716 mm TL, Banks Strait, Tasmania, ~ 40° S, ~ 148° E, 29 Jul. 2004; CSIRO H 6309–03 (skeletal parts), adult male 902 mm TL, east of Flinders Island, Tasmania, ~ 40° S, ~ 149° E, 400–450 m, 1 Aug. 2004; CSIRO H 6310–01 (skeletal parts), adult male 926 mm TL, CSIRO H 6310–02 (skeletal parts), male 882 mm TL, CSIRO H 6310–03 (skeletal parts), female 870 mm TL, northeast of Flinders Island, Tasmania, 39°04′ S, 148°39′ E, 500–680 m, 24 Jul. 1986; CSIRO H 6498–02 (skeletal parts), adult male 909 mm TL, off southern Flinders Island, Tasmania, ~ 40° S, ~ 148° E, 300–500 m, 24 Jun. 2003; CSIRO H 6499–01 (skeletal parts), female 1080 mm TL, CSIRO H 6499–02 (skeletal parts), female 880 mm TL, CSIRO H 6499–03 (skeletal parts), female 1070 mm TL, off northeast Tasmania, ~ 41° S, ~ 149° E, 24 Jul. 2003; CSIRO H 6503–01 (skeletal parts), adult male 872 mm TL, CSIRO H 6503–06 (skeletal parts), adult male 867 mm TL, northeast of Flinders Island, Tasmania, 39°20′ S, 148°45′ E, 370–420 m, 7 Apr. 2003.
Centrophorus moluccensis . CSIRO H 3599–04 (skeletal parts), adult male, southwest of Shark Bay, Western Australia, 27°05′ S, 112°45′ E, 303–333 m, 3 Feb. 1991.
Centrophorus squamosus . CSIRO H 1358–01 (skeletal parts), east of Dunk Island, Queensland Trough, Queensland, 18°08′ S, 147°11′ E, 200 m, 9 Dec. 1985.
TABLE 2. Cranial measurements of Centrophorus uyato, C. harrissoni, C. moluccensis and C. squamosus expressed as a percentage of neurocranium total length (% CL). N: number of specimens.
| C. uyato | C. harrissoni | C. moluccensis | C. squamosus | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min. | Max. | Mean | N | Min. | Max. | Mean | N | CSIRO H 3599-04 | N | CSIRO H 1358-01 | |
| Total length of neurocranium (CL) (mm) | 9 | 107.2 | 157.7 | 140.6 | 16 | 101.6 | 181.7 | 147.3 | 1 | 108.0 | 1 | 180.9 |
| Postcerebral length | 9 | 60.8 | 66.7 | 63.7 | 16 | 57.1 | 61.9 | 59.5 | 1 | 65.4 | 1 | 62.8 |
| Precerebral fossa length | 9 | 27.8 | 32.3 | 30.6 | 16 | 33.6 | 39.7 | 36.9 | 1 | 30.5 | 1 | 31.9 |
| Precerebral fossa width | 9 | 9.4 | 13.1 | 10.9 | 16 | 9.3 | 12.7 | 10.8 | 1 | 9.2 | 1 | 11.6 |
| Width across nasal capsules | 9 | 40.8 | 47.0 | 44.3 | 16 | 36.9 | 44.9 | 42.4 | 1 | 38.9 | 1 | 46.0 |
| Interorbital width | 9 | 29.0 | 33.4 | 31.5 | 16 | 25.3 | 28.0 | 26.6 | 1 | 25.3 | 1 | 27.0 |
| Width across preorbital processes | 9 | 34.7 | 40.2 | 37.6 | 16 | 31.8 | 36.9 | 34.0 | 1 | 40.6 | 1 | 41.1 |
| Width across postorbital processes | 9 | 44.4 | 49.8 | 47.0 | 16 | 36.1 | 44.0 | 38.8 | 1 | 38.2 | 1 | 44.8 |
| Width across prootic processes | 9 | 40.0 | 45.2 | 42.3 | 16 | 35.3 | 40.9 | 38.0 | 1 | 41.2 | 1 | 46.0 |
| Nasobasal length | 9 | 61.6 | 65.2 | 63.4 | 16 | 48.0 | 58.2 | 53.2 | 1 | 63.0 | 1 | 62.5 |
| Rostral keel length | 9 | 38.2 | 42.5 | 40.5 | 16 | 46.7 | 54.9 | 50.3 | 1 | 43.2 | 1 | 40.5 |
| Subethmoidean width | 9 | 5.4 | 7.9 | 6.9 | 16 | 7.6 | 8.9 | 8.4 | 1 | 3.5 | 1 | 6.9 |
| Basal plate length | 9 | 33.4 | 40.2 | 36.3 | 16 | 28.8 | 35.2 | 32.1 | 1 | 36.1 | 1 | 36.4 |
| Distance between postorbital and prootic processes | 9 | 19.9 | 23.1 | 21.7 | 16 | 18.5 | 22.0 | 19.7 | 1 | 22.1 | 1 | 26.4 |
| Width of basal angle | 9 | 15.3 | 17.4 | 16.1 | 16 | 12.3 | 16.4 | 14.6 | 1 | 16.0 | 1 | 22.6 |
| Width across 1st cartilaginous process | 9 | 25.0 | 28.3 | 26.5 | 16 | 23.1 | 26.9 | 24.9 | 1 | 26.9 | 1 | 30.0 |
| Width across 2nd cartilaginous process | 9 | 30.2 | 34.4 | 32.0 | 16 | 27.5 | 30.6 | 28.7 | 1 | 31.2 | 1 | 34.8 |
| Maximum sagittal height | 9 | 26.3 | 29.2 | 27.7 | 16 | 23.6 | 27.4 | 25.1 | 1 | 28.2 | 1 | 27.5 |
| Width of foramen magnum | 9 | 4.1 | 6.3 | 5.3 | 16 | 3.7 | 6.1 | 5.0 | 1 | 6.9 | 1 | 4.8 |
TABLE 3. Morphological measurements shown by SIMPER that contribute the most to differences between Centrophorus species. Pale orange cells are based on only size class 1 for each species; pale blue is based on the remaining larger size classes. * denotes those characters which differ from the corresponding size class for a particular species pair.
| C. granulosus | C. lesliei | C. longipinnis | C. uyato | |
|---|---|---|---|---|
| C. granulosus | Head width at ant. mouth First dorsal base | First dorsal base Head width at ant. mouth First dorsal length | Second dorsal soft length* Pectoral-pelvic space* First dorsal soft length* | |
| C. lesliei | Head width at ant. mouth First dorsal base First dorsal soft length* | First dorsal base First dorsal length First dorsal soft length | First dorsal base First dorsal soft length First dorsal length | |
| C. longipinnis | First dorsal base First dorsal length Head width at ant. mouth | First dorsal base Pectoral-pelvic space* First dorsal soft length | First dorsal base First dorsal soft length First dorsal length | |
| C. uyato | Pectoral inner margin* Interdorsal space* First dorsal base* | First dorsal base First dorsal length First dorsal length | First dorsal base First dorsal length First dorsal soft length |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Centrophorus uyato ( Rafinesque, 1810 )
| White, William T., Guallart, Javier, Ebert, David A., Naylor, Gavin J. P., Mo, Ana Veríssi-, Cotton, Charles F., Harris, Mark, Serena, Fabrizio & Iglésias, Samuel P. 2022 |
Centrophorus zeehaani
| White, Ebert & Compagno 2008: 1 |
Centrophorus zeehani
| White, Ebert & Compagno 2008 |
Centrophorus machiquensis
| Maul 1955: 5 |
Centrophorus cf. harrissoni
| McCulloch 1915 |
Centrophorus bragancae
| Regan 1906: 438 |
Centrophorus bragance
| Regan 1906 |
Acanthias nigrescens
| Nardo 1860: 70 |
Centrophorus (forme) uyato-machiquensis
| Muller & Henle 1837 |
Centrophorus ‘uyato’
| Muller & Henle 1837 |
Spinax uyatus
| Bonaparte 1834 |
Dalatias nocturnus
| Rafinesque 1810: 11 |
Squalus uyato
| Rafinesque 1810 |