Cryptomeria japonica
|
publication ID |
https://doi.org/10.1016/j.phytochem.2022.113520 |
|
persistent identifier |
https://treatment.plazi.org/id/0384AA0D-FF9D-FFF5-FC90-FE71FB9AFD2E |
|
treatment provided by |
Felipe |
|
scientific name |
Cryptomeria japonica |
| status |
|
2. C. japonica specialized metabolites
Owing to C. japonica ’ importance in the wood industry, the phytochemistry and bioactivities of its residues have been intensively studied for many years and still remain the focus of attention of many researchers, mainly from Asian countries, as summarized below and detailed in section 3.
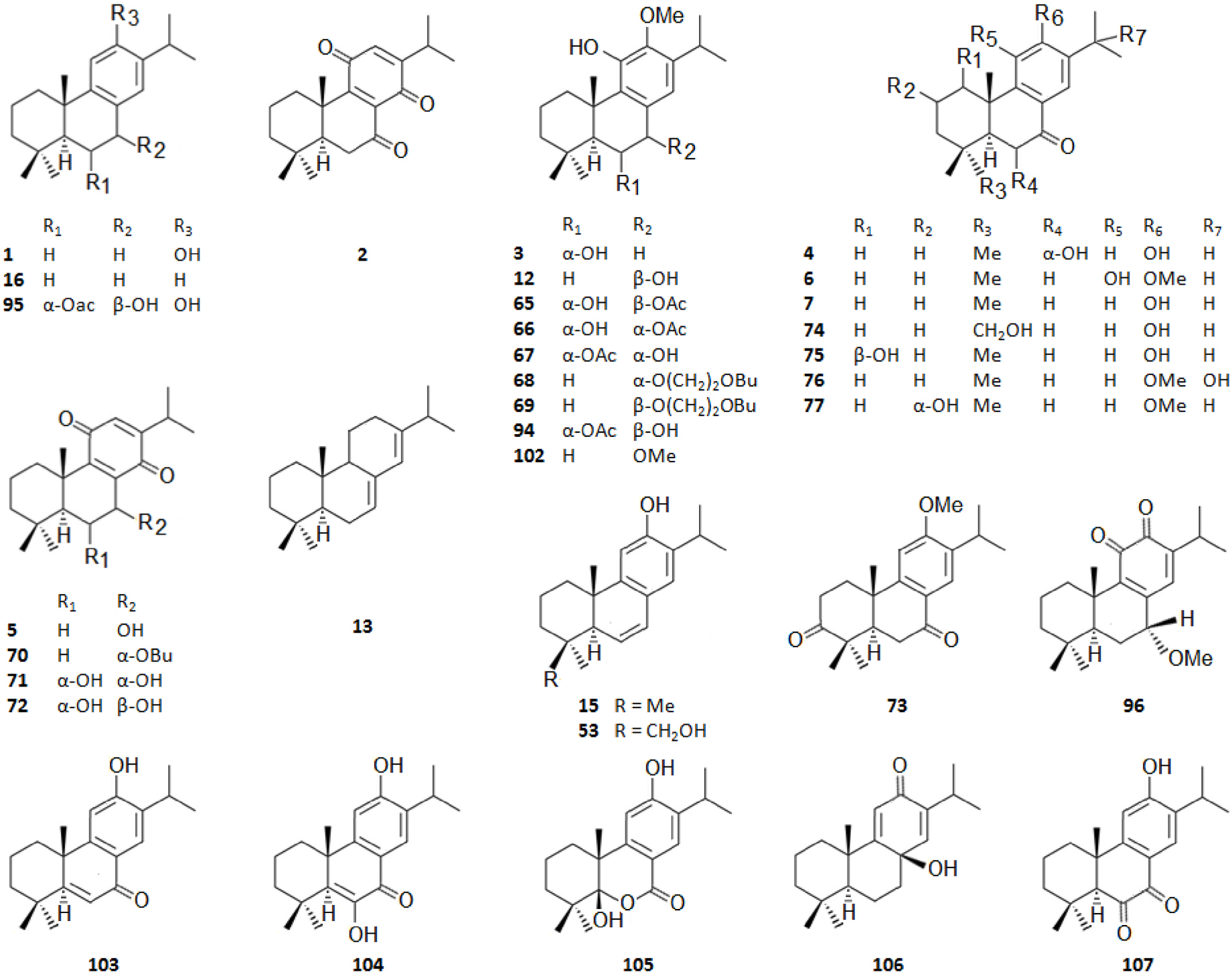
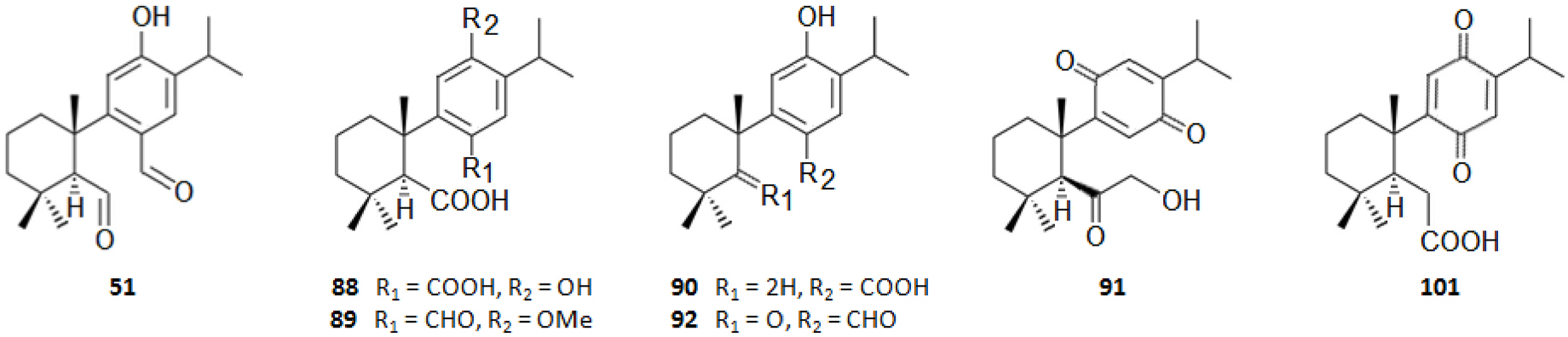
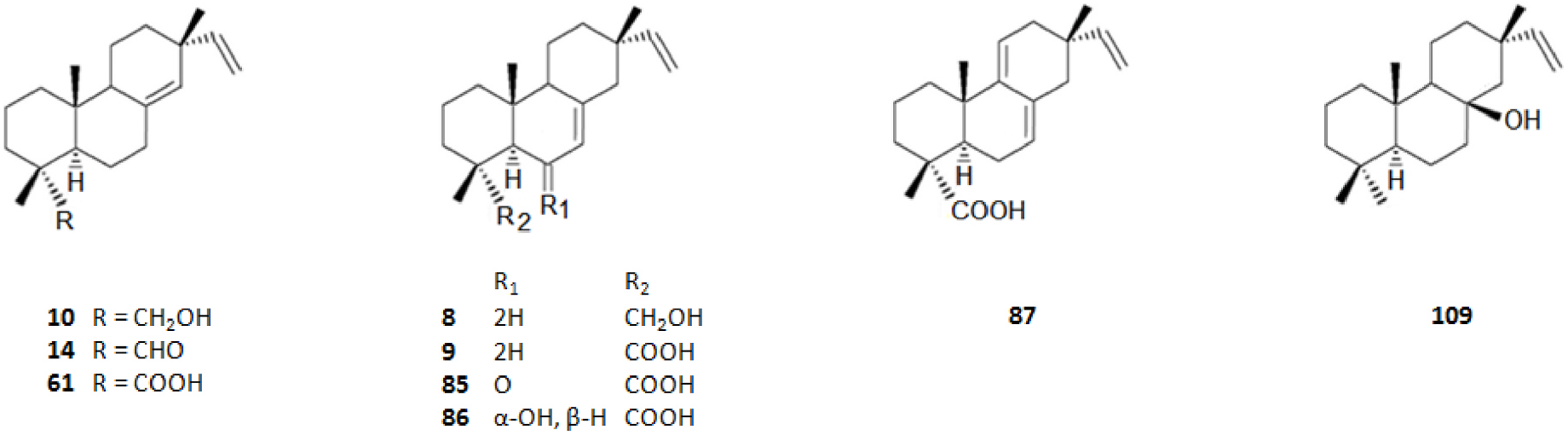
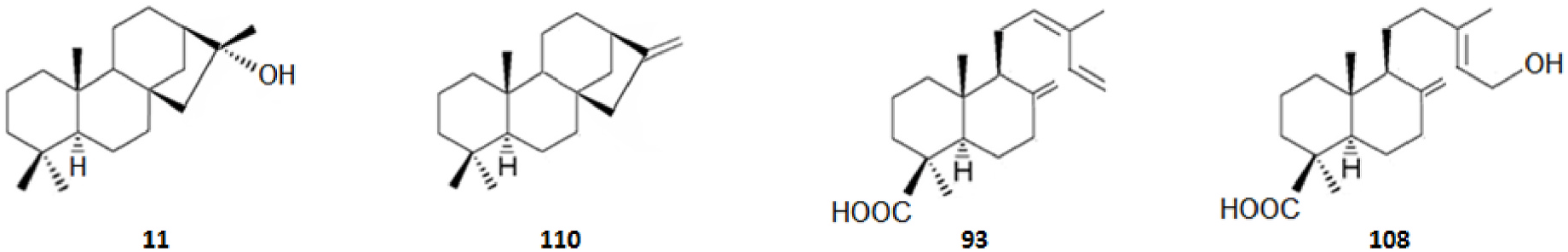
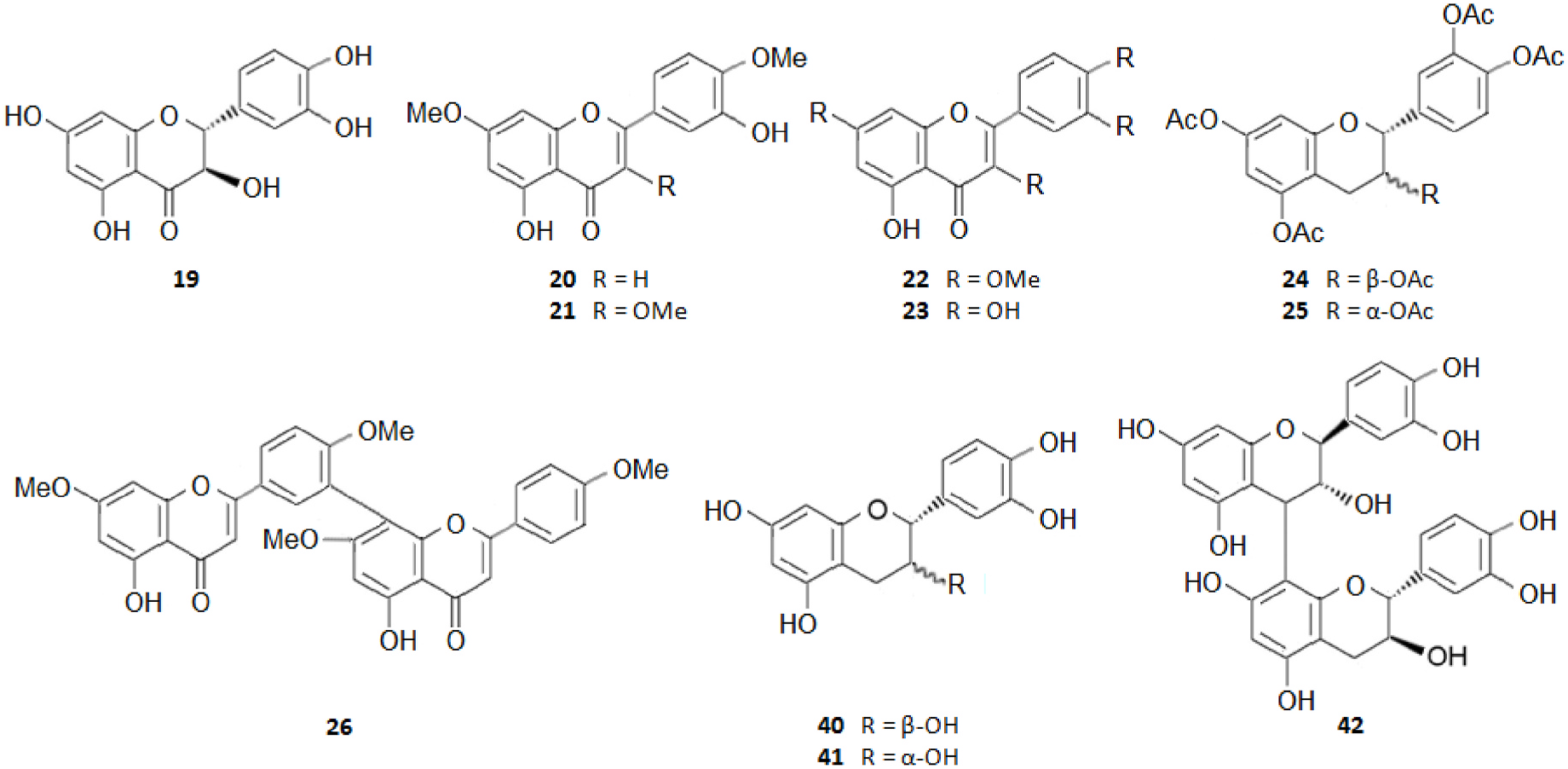
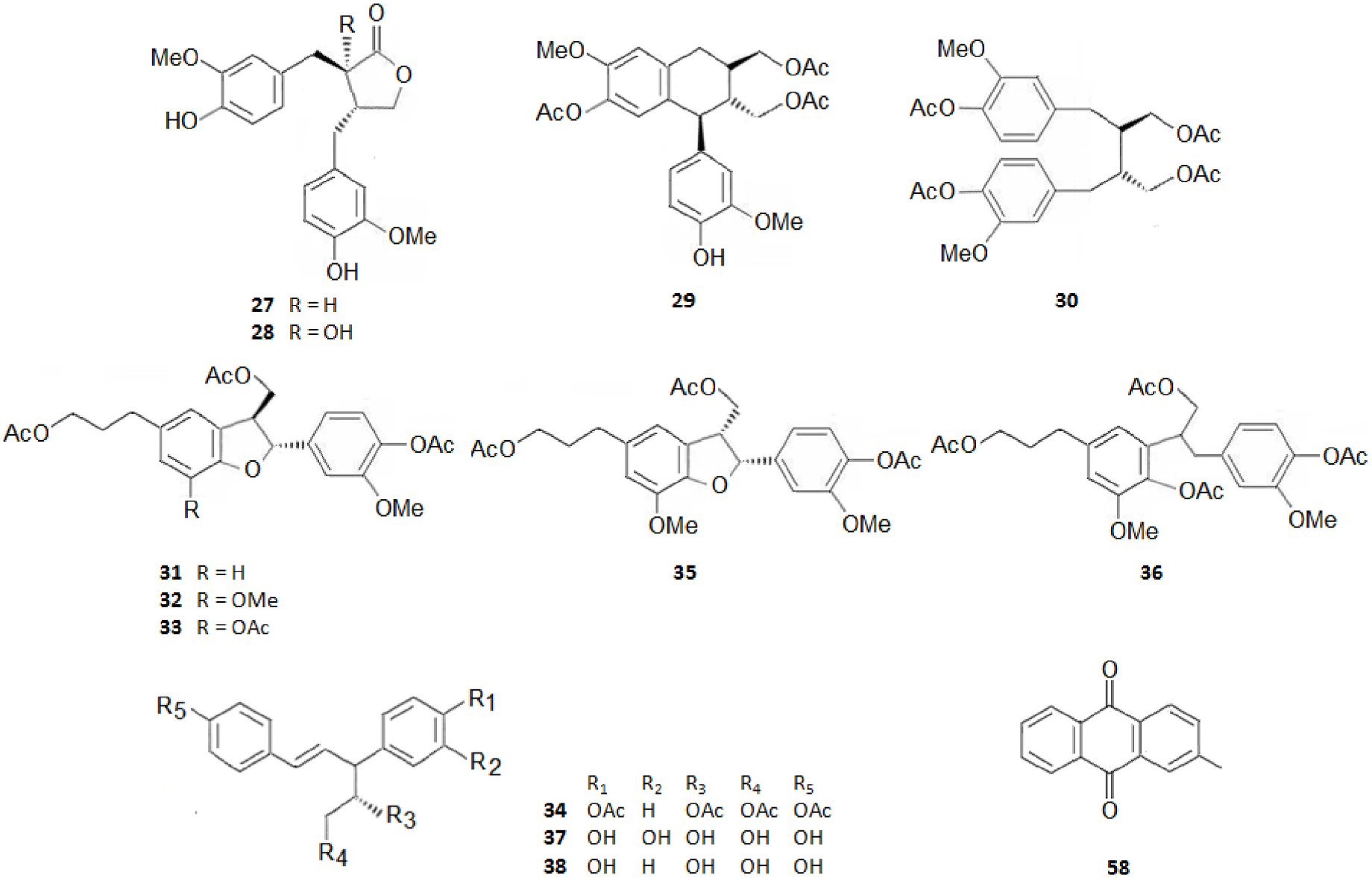
Among the innumerous C. japonica specialized metabolites, isolated since 1988 until now, we selected 118, as listed in Table 1, 50 of which were undescribed compounds that were reported within the covered period of time, and 63 possessed biological activities.
Table 2 shown the selected C. japonica specialized metabolites grouped according to their chemical class.
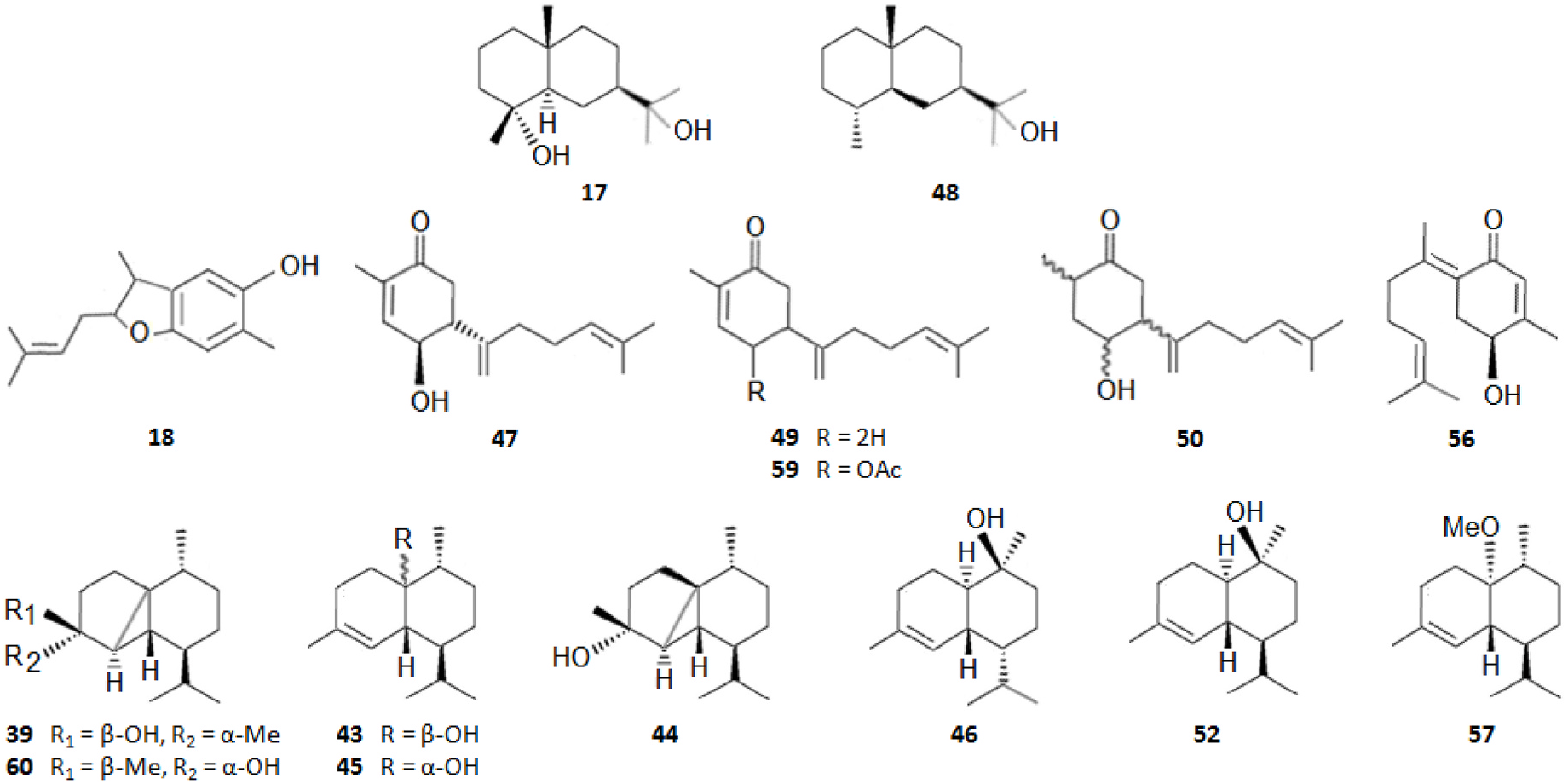
As summarized in Table 2, the extractable C. japonica specialized metabolites presented in this review are terpenes (79.7%) and polyphenols (20.3%). The terpenes include diterpenes/dimeric diterpenes (68.1%), sesquiterpenes (17%), triterpenes (8.5%) and sesquarterpenes (6.4%). The most widespread diterpenes (C20) are abietane/seco-abietane (76%) followed by pimarane/isopimarane (17%), and to a lesser extent kaurane and labdane-type diterpenes. The major sesquiterpenes (C15) are cadinanes (50%) followed by bisabolanes (37.5%), and to a lesser extent eudesmanes (12.5%). Concerning the polyphenols, the majority are flavonoids (45.8%) followed by lignans (37.5%), and to a lesser extent norlignans (12.5%) and anthraquinones (4.2%).
As also shown in Table 2, among the selected C. japonica specialized metabolites, 42.4% were undescribed compounds at the time of publication, and 53.4% are bioactive.
Figs. 2–11 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig below illustrate the chemical structures of all the specialized metabolites reported in the C. japonica research studies analyzed in the current review.
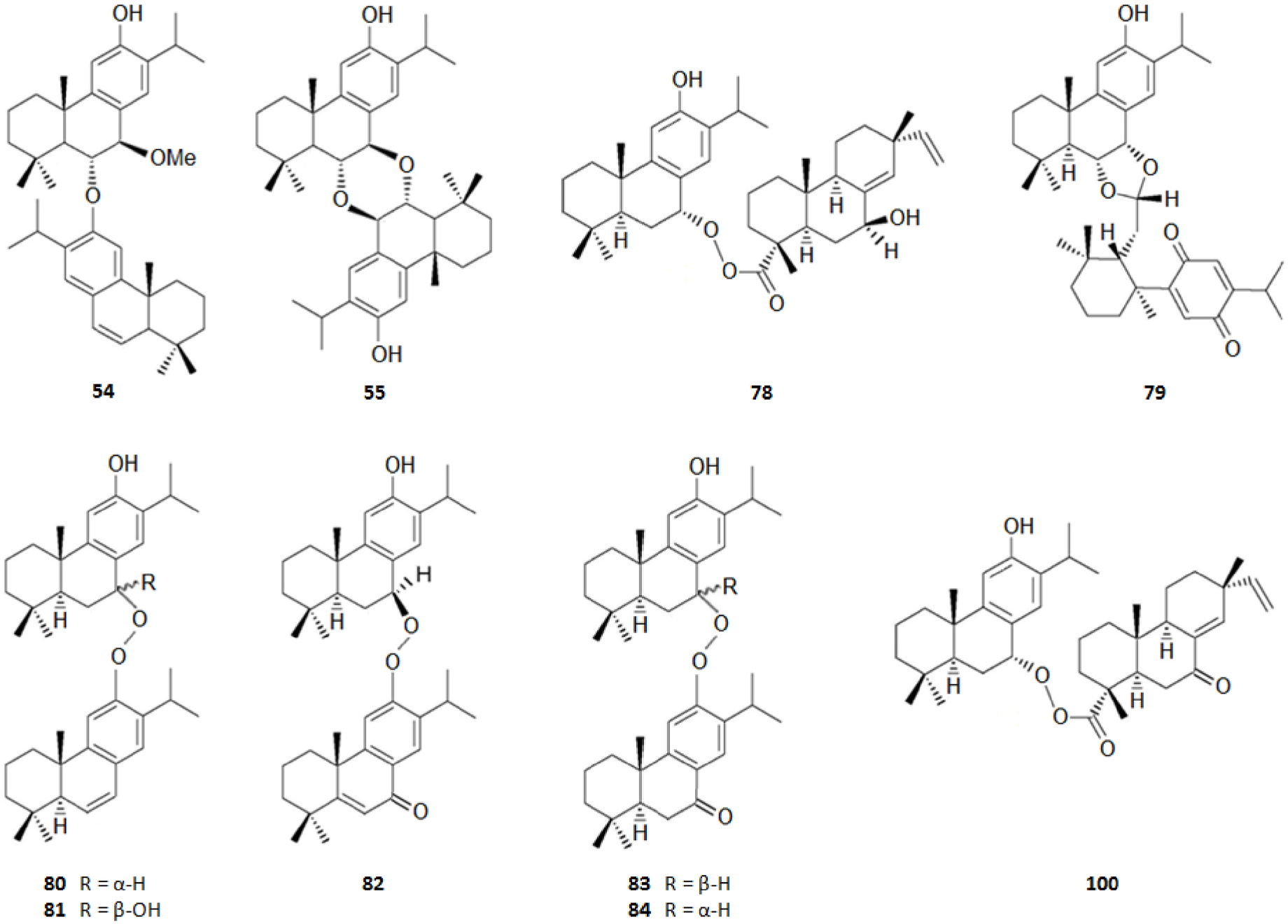
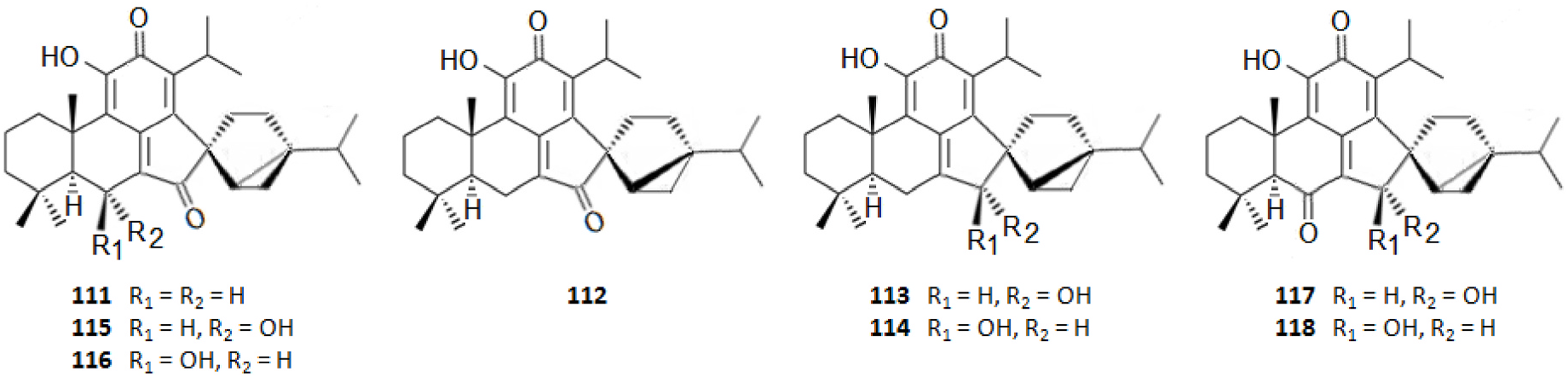
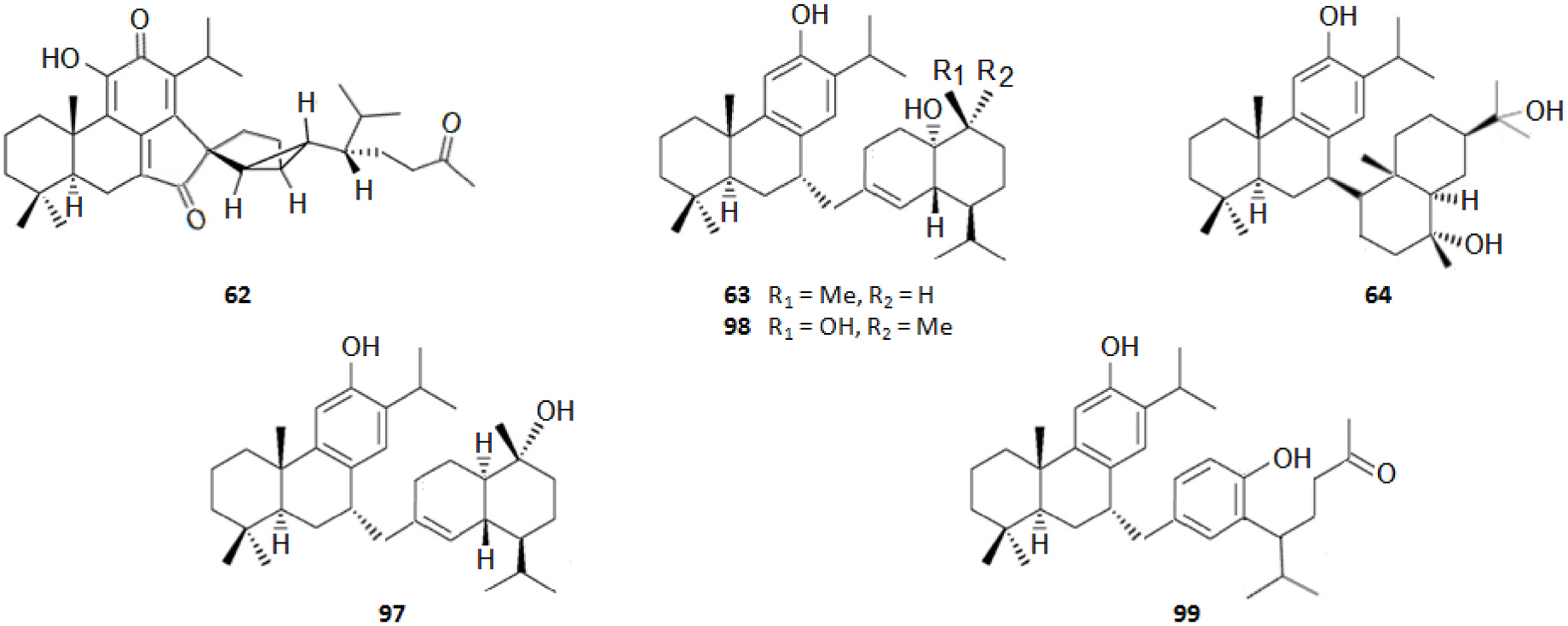
It should be highlighted that among the isolated compounds, there are interesting dimeric terpenes (i.e. mono-to-diterpenes, sesqui-toditerpenes and dimeric diterpenes), including the eight uncommon triterpenes ( Fig. 8 View Fig ), the six sesquarterpenes ( Fig. 9 View Fig ), which are abietane skeleton structures incorporating a sesquiterpene, and the ten dimeric abietane-type diterpenes ( Fig. 6 View Fig ). It should also be pointed out that the highly cytotoxic sesquarterpene cryptotrione ( 62), isolated from C. japonica bark in 2010 by the Kuo group ( Chen et al., 2010), is the structurally most complex member of cryptotrione family and its analogs, including the referred uncommon triterpenes 111–118 previously isolated by Shibuya (1992) and Su et al. (1993) from cones and leaves of C. japonica . These nine cryptotrione family members share a structurally unprecedented skeleton that comprises spiro-annulation of abietane-type diterpene and thujane-type monoterpene. Interestingly, the total synthesis of 62 was achieved recently by Lyu et al. (2020). As stated by the authors, their synthetic route would be practical for the synthesis of other members of cryptotrione family and its analogs for further pharmacological evaluation.
Table 3 shows the bioactive organic extracts/fractions and their specialized metabolites from different CJBR discussed in this review, as detailed in section 3.
As shown in Table 3, the bark, woody tissues and leaves are the main studied CJBR. However, despite less studied and available than other CJBR, the cones of C. japonica exhibited the C 30 -terpene quinone methides 111–115, two of which, named cryptoquinonemethides D ( 113) and E ( 114), were first isolated in this species ( Shibuya, 1992) and are regarded as precursors of chamaecydin ( 111). Other relevant terpenes, present in bark, are 2 and 3, and 94–101, isolated by Kofujita ( Kofujita et al., 2002, 2006) and Yoshikawa ( Yoshikawa et al., 2006a, 2006b) Japanese research groups, respectively, as well as 62–91 isolated by Kuo research group from Taiwan. Kofujita research group ( Arihara et al., 2004a, 2004c) has also isolated six relevant terpenes ( 18 and 53–57) from heartwood. Relatively to the leaf tissue, two lignans ( 35 and 36) were reported by Su et al. (1995). Thus, 50 undescribed specialized metabolites (48 terpenes and two polyphenols) were reported in the C. japonica research studies analyzed in the current review, mainly over the past three decades. Some of them showed remarkable anticancer activity and thus have attracted significant attention from organic chemists, such as cryptotrione ( 62) that displays a strong activity against human oral epidermoid carcinoma KB cells proliferation, with inhibitory concentration 50% (IC 50) value of 6.44 μM, comparable to that of the clinically used anticancer drug etoposide (VP-16, IC 50 = 2.0 μM) ( Chen et al., 2010).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.