Chelonia mydas
|
publication ID |
https://doi.org/10.1206/357.1 |
|
persistent identifier |
https://treatment.plazi.org/id/0385879E-473C-FFC7-3C71-9344FF3FFEDC |
|
treatment provided by |
Tatiana |
|
scientific name |
Chelonia mydas |
| status |
|
BERMUDA: Green turtles captured on the
Bermuda Platform over a 37-year period varied in length from 22.3–81.0 cm SCL. The smallest net capture was 22.3 cm SCL but individuals in the 25–30 cm size range were seen with much greater frequency than those in the 20–25 cm range (fig. 5 A). The mesh size of the entrapment net was 10 cm knot to knot ( 20 cm stretch mesh), suggesting that the minimum size of capture was not constrained by the entrapment net. Set nets were used to specifically address the question of whether larger turtles might be present but missed by the entrapment net. The four turtles caught by set netting were large (avg. 5 58.4 cm SCL), but the largest ( 67.3 cm) was much smaller than the largest turtle captured in the entrapment net. The most frequently represented size classes are in the 30–60 cm size range.
The stranded C. mydas from Bermuda with measurable SCL varied from 18.8– 97.7 cm (avg. 5 36.0 ± 12.8) and all were within the size range of netted individuals except for one 18.8 cm SCL immature and two adult-sized individuals. One of the adultsize turtles (estimated 90 cm SCL) was in a decomposed condition and its maturity status was not assessed. A 90 cm SCL green turtle is likely, but not certainly, mature (table 4; fig. 22). The other, a 97.7 cm SCL, 140 kg female, was found alive outside the reef line in June 1989. Damage to the shell suggested that she had been struck by a boat. This individual produced eggs while in rehabilitation. These are the only records of adult-sized green turtles in Bermuda recorded by the Bermuda Turtle Project .
The near absence of stranded C. mydas . 75 cm SCL is consistent with results from the netting work that suggest that C. mydas . 75 cm rarely occur in Bermuda waters or in the open ocean near the platform. The stranding network apparently does recover sea turtles that come from waters that are not sampled by the entrapment net. From July 1983 through December 2005, 62 Caretta ( 6.3–74.5 cm SCL, mostly posthatchlings) and three Dermochelys ( 116.8–146.3 cm SCL) were recovered. These species have never been captured in the entrapment net and are likely to come from off the Bermuda Platform. Similarly, the 18.8 cm SCL green turtle recovered by the stranding network is likely to have come from off the Bermuda Platform. Thus, despite sampling widely across the Bermuda Platform (fig. 2) with nets for 37 years, measuring a large sample of stranded individuals (fig. 5C) and conducting limited sampling with large-mesh set nets, only two adult-sized green turtles were recorded.
The smallest size of green turtles at Bermuda corresponds well to sizes at which C. mydas first appear at most other foraging grounds in the Western Atlantic (table 10). Among other benthic developmental stage studies in the literature, the site at which the smallest green turtle ( 20.8 cm SCL) has been captured is a cooling water intake at a power station in Florida ( Ernest et al., 1989; Bresette et al., 1998). The continental shelf is relatively narrow at this location and the water intake system for the plant could capture animals from the epipelagic stage. Other sites listed in table 10, including Secretary and Zapatilla, Panama; Cedar Key, Florida; St. Croix, U.S. Virgin Islands; Fernando Noronha, Brazil; and three Pacific sites, have larger minimum sizes. For the two Panama sites presented here, the most likely explanation for the larger minimum size is that immature individuals are recruiting to these sites from other benthic developmental habitats and not from the epipelagic stage. However, in Brazil, and even more likely in the Pacific, immature C. mydas may recruit from the epipelagic stage to benthic developmental habitats at a larger size than that seen in Bermuda and other Atlantic sites.
Given Bermuda’s mid-Atlantic location, the smallest of the green turtles seen on the Bermuda Platform have to recruit from adjacent epipelagic habitat. These small turtles have a bright white, rather than yellow, plastron and some carry goose barnacles ( Lepas ) that are associated with the pelagic environment. There is evidence
TABLE 10
Summary of foraging ground data for Chelonia mydas from the literature
Residency
Sample Size Mature Maturity or site Duration Capture
Location Habitat type size range (cm) ( %) criteria fidelity of study method Reference
Atlantic Ocean
Bermuda sea grass beds 2512 22.3–81 SCLmin 0.0 laparoscopy yes 32 yr entrapment this paper
and size net
Panama: Secretary sea grass beds 132 46.7–88.0 SCLmin 0.0 laparoscopy yes 10 yr set nets this paper
and size
Panama: Zapatilla grass beds and 92 49.3–92.5 SCLmin 46 a laparoscopy yes 10 yr set nets this paper
(immatures) reefs and size
Panama: Zapatilla grass beds and 79 79.4–105 SCLmin laparoscopy no 10 yr set nets this paper
(adults) reefs and size
Florida: Mosquito sea grass beds 94 29.5–75.4 SCLn-t 0.0 size yes 2.5 yr set nets Mendonca and
Lagoon Ehrhart, 1982
Florida: Indian sea grass beds 907 24.3–72.4 b SCLn-t 0.1 size yes 14 yr set nets Ehrhart et al.,
River Lagoon 1996
Florida: Indian sabellariid worm 190 25.1–67.0 SCLn-t 0.0 size yes 6 yr set nets Ehrhart et al.,
River reefs reefs 1996
Florida: Indian sea grass beds 342 24.6–75.4 b SCLn-t 0.3 tail size yes 5 events cold stunning Witherington and
River Lagoon Ehrhart, 1989
Florida:Indian various 246 26.6–77.0 SCLn-t 0.0 6 days cold stunning Schroeder and
River Lagoon Owens, 1994
Florida:Cape ship channel 20 23.5–68.1 SCLmax 0.0 size no 6 yr shrimp trawl Henwood and
Canaveral Ogren, 1987
Florida: NE coast longshore 41 24.0–55.4 SCLn-t 0.0 Size no 6 yr shrimp trawl Schmid, 1995
Florida: Broward nearshore hard 187 27.4–67.0 CCL 0.0 Size yes 5 yr dive / snorkle Wershoven and
County bottom Wershoven, 1992
Florida: St. Lucie nearshore cold 1673 20.8–105.3 1.1 Size yes 22 yr power plant Ernest et al., 1989 ;
Co. water intake SCLmin intake Bresette et al., 1998
Florida: Florida Bay inshore 69 25.5–66.1 SCLn-t 8 yr nets/rodeo Schroeder et al., 1998; Schroeder, personal commun.
Florida: Cedar Key sea grass flats 198 34.6–76.3 SCLn-t 0.0 Size yes 8 mo set nets Carr and Caldwell, 1956
Florida: St. Joseph 389 26.2–80 CCLn-t cold stunning Foley et al., 2007
Bay
that green turtles use the sargassum mats in the Sargasso Sea during the epipelagic stage that precedes recruitment into a neritic lifestyle ( Carr, 1987). Bermuda is located in the northern portion of the Sargasso Sea, so epipelagic C. mydas in the Sargasso Sea may need to travel only a short distance to reach suitable benthic foraging grounds.
A contingency test indicates a significant difference in the size distribution of stranded turtles relative to that of live captures on the Bermuda Platform (x 2 5 298.8, 15 df, P, 0.05). One factor contributing to this difference is the relatively large proportion of small turtles present in the stranded sample. Turtles in the 20–30 cm SCL size class made up 39 % of the stranded sample compared to 7.8 % of the live captures. Assuming that stranding records accurately reflect mortality patterns, it could be inferred that there is disproportionately high mortality associated with green turtles in the size class that is recruiting from epipelagic into benthic developmental habitat.
Laparoscopy of 131 C. mydas in Bermuda ( 54 males, 77 females; figs. 5B, 6) did not reveal any mature individuals. Twenty-one (16.0 %) of the laparoscoped turtles showed evidence of puberty, either ridged epididymides in males or differentiation of follicle size in females ; but none showed the enlarged testis or pendulous epididymis of adult males or the enlarged follicles and wide oviduct of adult females ( Limpus and Reed, 1985a). Thus, it appears that green turtles leave Bermuda before they reach maturity (fig. 5).
The green turtles that occupy the Bermuda Platform appear to be exclusively postpelagic immatures living in an area where adults are absent. Green turtles have received complete protection from harvest in Bermuda since 1973, and there has been ample time for the largest green turtles observed during the early years of this study (first records are from 1968) to mature in Bermuda, if that were the normal pattern. Laparoscopic data from this study indicate that C. mydas may begin puberty in Bermuda, but that they go elsewhere to complete the maturation process. The absence of adults, as seen in Bermuda, appears to be a characteristic of a majority of benthic developmental habitats for C. mydas , at least in the Atlantic.
PANAMA: SECRETARY AND ZAPATILLA CAYS STUDY SITES: The size distributions of samples at the Secretary and the Zapatilla Cays study sites likely reflect the true range of sizes present at those sites. If larger turtles were present at the Secretary site, they would be captured because the nets used at Secretary are routinely used to catch adult green turtles at the Zapatilla site (see below). Concerning smaller turtles, while it is possible that individuals under about 35 cm could pass through the larger mesh nets ( 40 cm bar mesh), nets with 20 cm bar mesh have also been used at Secretary. Other investigators using nets with a 20 cm bar mesh size catch green turtles as small as 24.3 cm SCL, and have hundreds of captures in the 30–40 cm SCL range ( Ehrhart et al., 1996, 2007). At Secretary, limited testing was also done with a net with a 10 cm bar mesh; no turtles were captured. Nets with this very small mesh size were not routinely used because of bycatch problems.
At both of the Panama study sites, C. mydas were observed for the first time in the nets at a larger size than in Bermuda. The smallest were 46.7 cm SCL at Secretary, and 46.2 cm SCL at Zapatilla (excluding one 29.3 cm outlier; fig. 14B), compared to 22.3 cm SCL in Bermuda. Because the nets that were used at both sites in Panama could have caught smaller green turtles, it seems likely that smaller green turtles were not present at either site. Discussions with turtle fishermen indicated that there are sea grass beds elsewhere in Bocas del Toro Province where smaller green turtles do occur. Results presented here from Bermuda and the preponderance of evidence from the literature (table 10) suggest that the most likely explanation for the larger minimum size in the Panama sites is that the smallest green turtles have moved into those sites after spending time in the benthic developmental stage elsewhere.
Of the 132 C. mydas observed at Secretary, only 14 were larger than the minimum size at sexual maturity observed for this species in this study. Seven of these 14 were found to be immature via laparoscopy; the maturity status of the remaining 7 (5.3 %) was not determined. Thus, Secretary appears to serve primarily, and perhaps exclusively, as benthic developmental habitat for this species.
The size distribution data for C. mydas at the Zapatilla Cays (fig. 19) are more complex than those for Bermuda or Secretary. From approximately April to September, large numbers of adult green turtles (maturity based on laparoscopy, size, presence of secondary sexual characteristics, and tag returns from the nesting beach) were observed at this site. Interviews with fishermen, tags on turtles captured at the study site, foreign tag-return data, genetic data, and satellite telemetry all suggest that these adults are migrating to Tortuguero, Costa Rica (Meylan and Meylan, unpubl. data). However, not all of the C. mydas at the Zapatilla site are mature. Among the 265 individuals captured, at least 128 were immature.
Green turtles were classified as mature or immature using both laparoscopy and size criteria. C. mydas with stage 1 or stage 2 gonads were considered immature, as were individuals that had not been laparoscoped but were less than 76.7 cm SCL. The size criterion was based on the observation that the smallest mature green turtle examined via laparoscopy in Panama was 76.7 cm SCL ( n 5 178) (this study; Meylan and Meylan, unpubl. data). Using these two criteria allowed most turtles to be reliably assigned. However, there were undoubtedly additional immature animals in the sample that were larger than 76.7 cm that were not laparoscoped and may have been incorrectly categorized as adults. However, relatively few individuals fall into this category. Adult turtles were likely to be correctly classified as mature in nearly all, if not all, cases because of the very conservative minimum size at maturity that was used.
The size distribution of immature green turtles at the Zapatilla study site is given in figure 14B. This subset of turtles showed a different pattern of habitat use as well as arrival and departure behaviors than adults (see below). The best explanation for the observations made at the Zapatilla Cays is that this area serves as benthic developmental habitat for large immatures and is shared seasonally with migratory adults (figs. 19 A, 23 A). If the single, nonmigratory sample from January is representative, the Zapatilla foraging ground is occupied almost exclusively by immatures, except during the reproductive season.
THE LITERATURE: Including the results presented here, we are aware of 22 foraging ground studies of C. mydas in the Atlantic system with results that are consistent with the existence of a discrete benthic developmental stage for this species (table 10, fig. 24). These studies were conducted over a wide range of localities and with a variety of capture methods. They included data on thousands of green turtles that were considered immature, usually on the basis of size. The smallest C. mydas at these study sites varied from 20.8–46.2 cm SCL and the largest immatures varied in size from 52.0– 81.5 cm. Only four of these studies reported observations of mature individuals in their samples and when adults were present, they made up # 1.1 % of the sample. In some of these cases, turtles that were actually immature may have been considered mature because they were larger than some minimum size of sexual maturity. In one study in which adults were captured along with immatures ( Ernest et al., 1989; Bresette et al., 1998), nesting habitat for C. mydas is nearby and internesting habitat could be expected to overlap with benthic developmental habitat (fig. 23B).
Benthic developmental habitat appears to be used seasonally by green turtles along the east coast of the United States, north of Florida ( Hillestad et al., 1978; Lazell, 1980; Epperly et al., 1995; Barnard et al., 1989; Morreale et al., 1992; McClellan and Read, 2009). However, green turtles appear at these localities in small numbers and do not represent a significant portion of the individuals that live in the North Atlantic. This differs from the case for Lepidochelys kempii and Caretta caretta (see below) for which seasonal use of benthic developmental sites along the U.S. east coast appears to be more frequent.
Most foraging grounds for C. mydas in the Pacific have both subadults and adults present at the same locality. These examples are presented in a section on contradictory evidence (see below). There are, however, a few Pacific sites at which immatures were reported to predominate, including Palaau, Hawaii ( Balazs et al., 1987) and Wuvulu Island, New Guinea ( Hirth et al., 1992). At the foraging area at Palaau, Hawaii, Balazs et al. (1987) reported 133 individuals between 35 and 80 cm SCL (fig. 24C). However, they added that larger turtles occur at this site at times and/or that larger turtles may have been caught there historically. In his synopsis of the biology of C. mydas in the Hawaiian Islands, Balazs (1980) suggested that immatures frequent the same areas as adults. However, he reports that there is a tendency for immatures to use shallower resting sites and that they sometimes use feeding pastures that are too shallow for adults. Thus, any isolation of immatures from adults in Hawaii may occur on a very local scale.
Hirth et al. (1992) observed 173 C. mydas , none of which they considered mature, during a brief visit at Wuvulu Island, New Guinea. A subsample of 34 turtles that were captured and measured ranged in size from 36.8–76.2 cm SCLn-t. They also reported that sea turtles at this island have been protected for religious reasons since 1952. This implies that if mature turtles used this site historically, the population has had sufficient time to recover from any previous harvest and adults should have been observed. Thus, this site is an example of benthic developmental habitat for green turtles in the Pacific at which adults appear to be absent.
In summary, ample data support the existence of a benthic developmental stage in the life cycle of C. mydas at 22 different foraging grounds in the North and South Atlantic, the Caribbean, and the Pacific. However, there is some contradictory evidence from this species in the Pacific, which is discussed below.
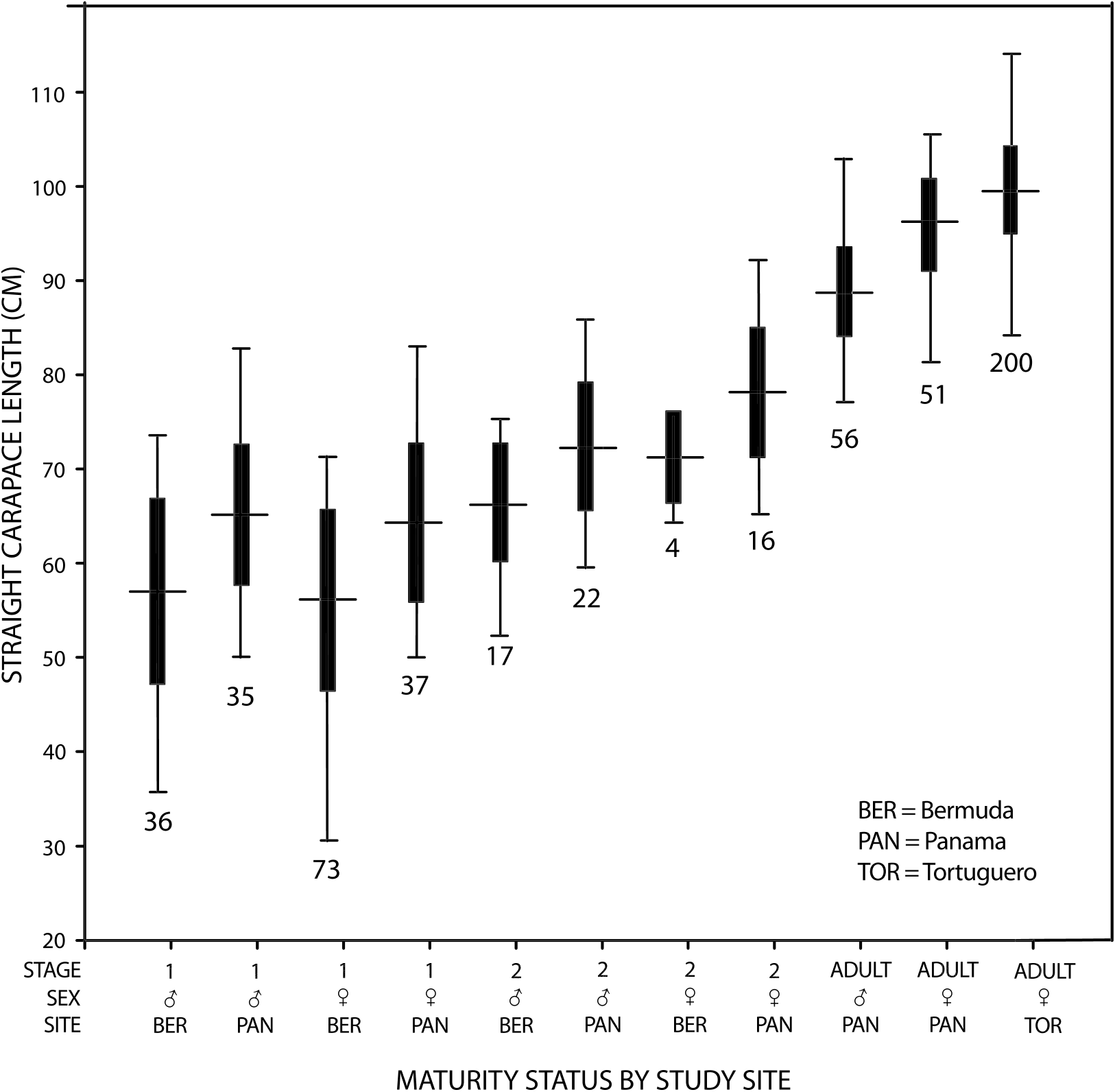
PUBERTY IN CHELONIA MYDAS IN THE ATLANTIC: Laparoscopic examination of large numbers of C. mydas in Bermuda and Panama provides information concerning the onset of maturation (puberty, following Limpus and Reed, 1985a) and the attainment of maturity in this species in the Atlantic. Figure 22 View Fig and table 4 show the mean size and size range by sex for three maturity stages of C. mydas from Panama and Bermuda. A sample of 200 nesting females from Tortuguero, Costa Rica, is included for comparison. Departure by green turtles from Bermuda by about 75 cm SCL truncates the size distribution of samples from this site. Nonparametric analysis of variance shows significant differences among the samples; howev- er, Dunn’s multiple comparison of means revealed relatively few significant pair-wise differences. Nesting females from Tortuguero and adult females from Panama were significantly larger than all stage 1 samples and stage 2 males from Bermuda; and adult males from Panama were significantly larger than all stage 1 samples.
Laparoscopic data from Panama and Bermuda suggest that puberty in Atlantic green turtles may begin at a smaller size in males than in females (see also figs. 6, 15, 20). The smallest male that showed signs of puberty was a 52.7 cm SCL individual from Bermuda, while the smallest female beginning to mature, also from Bermuda, was more than 10 cm larger, 64.5 cm SCL. Pubescent males (stage 2) from Bermuda (avg. 5 66.6 ± 6.4 cm SCL, n 5 17) were smaller on average than pubescent females (avg. 5 71.4 ± 4.8 cm SCL, n 5 4), but not significantly so. The small sample size for the slightly larger pubescent females is likely due to their departure from the study site.
Combining the Secretary and Zapatilla Cays laparoscopy data sets indicates that in Panama, too, male green turtles in the study reached puberty at a smaller average size (avg. 5 72.4 cm SCL) than females (avg. 5 78.2 cm SCL), but this difference was not statistically significant. Although differences between mean size of stage 2 males and females was not statistically significant (because of large variance), it may be biologically significant. If both sexes grow at equal rates, and males begin sexual maturity at smaller sizes and mature at smaller sizes (minimum 76.7 cm SCL; avg. 5 89.0 ± 4.7 cm), then males may, on average, mature a few years before females (minimum 81.0 cm SCL; avg. 5 95.9 ± 4.9 cm). Sexual size dimorphism in C. mydas has also been reported from Ascension Island ( Godley et al., 2002), so this pattern may extend beyond our Panama study .
Puberty was observed at sizes as small as 52.7 cm SCL in male C. mydas in Bermuda, but had not begun in an 83.4 cm SCL male from Panama (fig. 20). Puberty was observed in females as small as 64.5 cm (in Bermuda) but had not begun in an 86.2 cm SCL female from Panama. Thus, the onset of puberty, like the attainment of maturity, occurs across a wide range of sizes in this species. This illustrates the problem of using a minimum size criterion for maturity. Although the smallest mature male observed in Panama was 76.7 cm SCL, three (11.1 %) of 27 males from Secretary determined by laparoscopy to be immature, and eight (20 %) of 40 immature males from the Zapatilla Cays were larger than this minimum size. Similarly, three (11.5 %) of 26 immature females from Secretary and three (10.0 %) of 30 immature females from the Zapatilla Cays were larger than the smallest mature female (81.0 cm). This overlap in size ranges between immature and mature green turtles could lead to significant errors in the estimation of the number of mature animals at a site if size alone is used as the maturity criterion.
| SCL |
St. Cloud State University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |