Oenochroma barcodificata Hausmann & Young
|
publication ID |
https://doi.org/10.5281/zenodo.190505 |
|
DOI |
https://doi.org/10.5281/zenodo.6217087 |
|
persistent identifier |
https://treatment.plazi.org/id/0386050A-FF98-FF94-FF4E-FC91F672F98E |
|
treatment provided by |
Plazi |
|
scientific name |
Oenochroma barcodificata Hausmann & Young |
| status |
sp. nov. |
Oenochroma barcodificata Hausmann & Young , sp. nov.
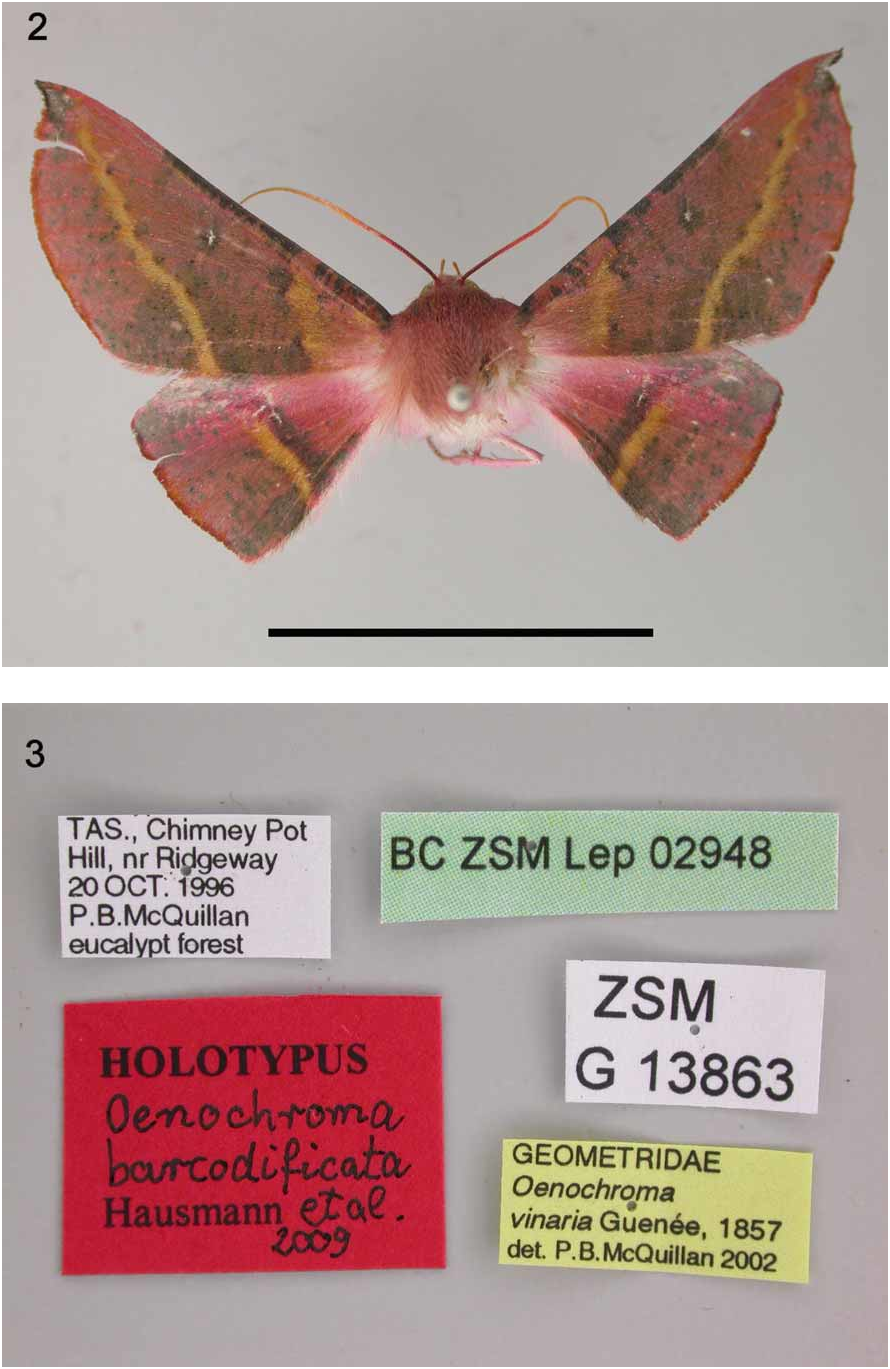
Type material. Holotype: Ƥ, ‘TAS[ MANIA], Chimney Pot Hill, nr Ridgeway, 20 OCT. 1996, eucalypt forest, leg. P.B. McQuillan’, coll. TMAG, genit.slide ‘ ZSM G 13863’, specimen ID ‘ BC ZSM Lep 0 2948 ’ [sequence ID GWORB 1068-07]. Paratypes: coll. BIO: 63 Australia, Tasmania, Hobart, Kingston Beach, lat. -42.986 x long. 147.317, alt. 110 m., 0 2., 11. and 18. XII.2005, 10. X.2006, 27. XII.2006, 22. I.2007, leg. R. D. Ward, sequence IDs: LOTSA 134-06/06-TASA-00134, LOTSA 125-06/06-TASA-00125, LOTSA 122-06/06-TASA- 0 0 122, LOTSC 391-07/06-TASB-01390, LOTSC 814-07/06-TASB-01813, LOTSD 507-08/07-TASB-0507; coll. M. Sommerer: 1Ƥ, Tasmania sept., vic. Mt. Roland, Silver Ridge Lodge, 41°27’ S / 146°15’ E, 320 m, 25-28.I.2006, leg. M. Sommerer, BC ZSM Lep 0 8033. coll. ANIC (153, 16Ƥ): 13 ‘Mt Wellington 870m [Tas.] 6 Dec. 1980 L. Hill’; 13 ‘ 43.03S 146.17E Huon Camping Area Tas. 25 Jan 1983 J.C. Cardale’; 13 ‘Lake Fenton 1060m Tas. 14 Jan 1978 P.B. McQuillan’ ‘ ANIC Genitalia slide 18601’; 13 ‘Mt Read nr Rosebery Tas. 4–5 Dec 1990 P.B. McQuillan’; 13 ‘ 41.51S 145.33E Mt Read Tas. 500m 9 Jan 1989 P.B. McQuillan E.S. Nielsen’; 13 ‘ 43.25S 146.09E Melaleuca Tas. 15 Jan 1991 E.S. Nielsen E.D. Edwards’; 23 ‘ 43.25S 146.09E Melaleuca Tas. 28 Nov 1991 E.S. Nielsen M. Horak’; 13 ‘ 43.25S 146.09E Melaleuca Tas. 25 Nov 1991 E.S. Nielsen M. Horak’; 13 ‘ 43.25S 146.09E Melaleuca Tas. 14 Jan 1991 E.S. Nielsen E.D. Edwards’; 13 ‘ 43.25S 146.09E Melaleuca Tas. 17 Jan 1991 E.S. Nielsen E.D. Edwards’; 13 ‘ 43.23S 146.08E Claytons Bathurst Harb. Tas. 16 Jan 1991 E.S. Nielsen E.D. Edwards’; 1Ƥ ‘Mt Wellington 1000m [Tas.] 8 Dec 1980 L. Hill’; 1Ƥ “Mt Wellington 870m [Tas.] 6 Dec 1980 L. Hill’; 1Ƥ “ 42.02S 146.33E 12km NNE Bronte Park Tas. 20 Jan 1983 J.C. Cardale’; 1Ƥ ‘ 42.02S 146.33E 12km NNE Bronte Park Tas. 2 Feb 1983 J.C. Cardale’; 2Ƥ “Pensford Tas. 920m [Tas.] 22 Dec 1981 L. Hill’; 1Ƥ ‘ 40.58S 148.01E 1km SSE Gladstone Tas. 29 Jan 1983 J.C. Cardale’; 1Ƥ ‘Mt Nelson Tas. 160m 3 Nov 1979 P.B. McQuillan’; 1Ƥ ‘ 41.51S 145.33E Mt Read 500m Tas. 9 Jan 1989 P.B. McQuillan E.S. Nielsen’; 1Ƥ ‘ 43.25S 146.09E Melaleuca Tas. 14 Jan 1991 E.S. Nielsen E.D. Edwards’; 1Ƥ ‘ 43.23S 146.08E Claytons Bathurst Harb. Tas. 16 Jan 1991 E.S. Nielsen E.D. Edwards’; coll. TMAG: 13 ‘Tas. Pt. Sorell. Tas. U. V. L. 14.x.1988 L. Hill’ ‘Registration No. F4702’ ‘ TMAG genitalia slide F4703’; 13 ‘ 41°52’S 146°30’E TAS. Central Plateau L. Augusta to L. Ada 1150 m 3 FEB. 1994. P. B. McQuillan uvl’ ‘Registration No. F4704’ ‘ TMAG genitalia slide No. F4705’; 13 ‘ 41°52’S 146°30’E TAS. Central Plateau L. Augusta to L. Ada 1150 m 3 FEB. 1994. P. B. McQuillan uvl’ ‘Registration No. F4706’ ‘‘ TMAG wing slide No. F4707’; 13 ‘S.W. TAS. 12 Trees Hill (Strathgordon) U.V.L. 17.xi.1989. T. Semmens’ ‘Registration No. F4708’ ‘ TMAG genitalia slide F4709’; 13 ‘40°6’8.53”, 148°0’15.07” Bluff Rd. Whitemark Flinders Is. Tas. 31 Oct. 2008 C. J. Young U.V.L’. ‘Registration No. F4710’; 1Ƥ ‘41°11’5”, 146°19’5” Stony Rise Centre, Devonport. Tas. 23–29 Sept. 2004 L. Hill’; coll. ZSM: 1Ƥ S.W. TAS. 12 Trees Hill (Strathgordon) U.V.L. 17.xi.1989. T. Semmens’ ‘Accession No. 104062’; 1Ƥ ‘Mt Wellington 870m [Tas.] 12V UV 3 Nov. 1981 L. Hill’; 23 ‘40°6’8.53”, 148°0’15.07” Bluff Rd. Whitemark Flinders Is. Tas. 31 Oct. 2008 C. J. Young U.V.L’. ‘Accession Nos 104058, 104060’.
Other material examined: New South Wales, coll. ANIC (all excluded from the type series): 1Ƥ ‘Newnes S[tate].F[orest]. [ NSW] 20 Nov 1993 J.C. Keast’; 1Ƥ ‘ 36.28S 148.27E Rangers Stn 6km NE by E Thredbo NSW 1260m 6 Jan 2002 E.D. Edwards’; 1Ƥ ‘ 36.23S 148.25E 1.5km NNW Smiggin Holes NSW 1700m 9 Jan 2002 E.D. Edwards’; 1Ƥ ‘ 36.23S 148.25E Saddle 2km NW Smiggin Holes Kosciusko Nat. Pk 1680m 23 Jan 1987 E.D. Edwards’; 1Ƥ ‘ 1.3 km E of Island Bend NSW 1158m 30 Nov 1970 I.F.B. Common J.S. Dugdale’; 23 ‘ 36.23S 148.22E Guthega Village Kosciusko N.P. 1700m [ NSW] 24 Jan 1987 E.D. Edwards’, one with ‘ ANIC genitalia slide 18603’; 13 ‘ 36.23S 148.25E Saddle 2 km NW Smiggin Holes Kosciusko N.P. 1680m [ NSW] 23 Jan 1987 E.D. Edwards’.
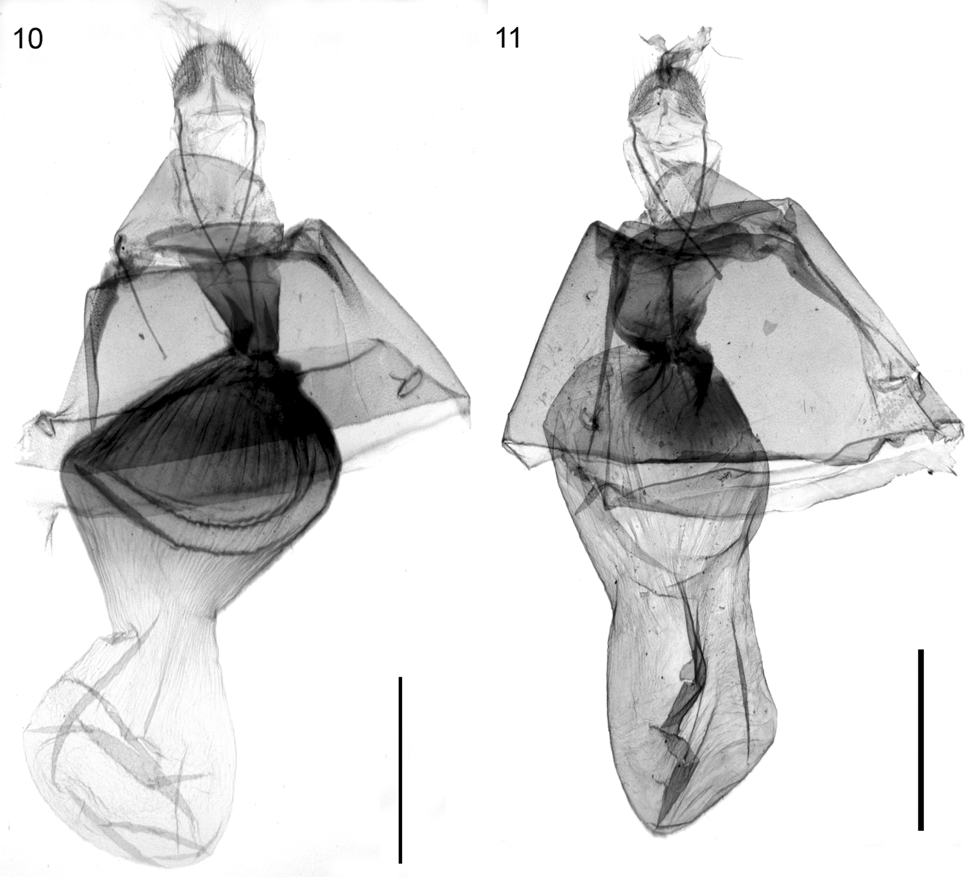
Description. Wingspan 3 42–47 mm. Habitus and external characters ( Figs. 2–3 View FIGURES 2, 3 ): Forewing apex tapered, termen convex. Hindwing termen straight, angled at apex and tornus. Ground colour variable, usually purplish with dark suffusion and with blackish dotting of forewing costa. Fringe crimson. Antemedial line of forewing vague, at costa usually well marked by a costal spot. Postmedial line of forewing almost straight but slightly undulating, ochre with dark grey dots basally, obliquely leading into forewing apex. Border of postmedial line on the hindwing more violet and forked towards costa. Forewing apex with blackish fringe. Cell spots slightly elongate, black, with small hyaline filling. Underside of forewing with a large, round dark violet spot close to the inner termen at 2/3, i.e. at the position of the dotted postmedial line. Underside of hindwing with large white and grey speckled spot close to the costa at 2/3, i.e. at the position of the dotted postmedial line. Palpi, frons, and vertex concolorous with ground colour. Frons flat. Palpi at upperside of tip with dark grey scales, last segment narrow, length of palpi ca 1.5 times diameter of eye in both sexes. Proboscis well developed. Antennal flagellum thick in both sexes, male antennae unipectinate to 2/3 of length, female antennae filiform. Frenulum strong in 3, absent from Ƥ. Wing venation, forewing: R separate from Rs, anastomosing with Sc for a short distance in the forewing, R2–5 stalked. Hindwing: M2 located at mid-point between M1 and M3. Hindleg with four very short spurs in both sexes. Ansa narrow at the base, widening mesally, tapering apically. Last abdominal segment with extremely large interior coremata. 3 genitalia ( Fig. 8 View FIGURES 8 ): Uncus long, narrow. Gnathos slender; medial process broad, sub-acute posteriorly, posterior surface covered with rows of short, broad spicules. Juxta large, well-sclerotised, divided. Saccus broad rectangular, with central invagination. Costa of valva sclerotised, setose. Valva asymmetrically adorned with subapical processes on ventral margin: left valva with single, large, sclerotised subapical, flattened, acute process, right valva with two short, broad processes. Aedeagus comparatively broad; vesica well sclerotised posteriorly, with longitudinal ridges; long narrow sclerotised process attached anteriorly, no discrete cornuti; caecum long, slender, tapered. Ƥ genitalia ( Fig. 10 View FIGURES 10, 11 ): Apophyses anteriores, posteriores long, slender. Lamella postvaginalis membranous. Lamella antevaginalis poorly developed. Sternum A7 slightly sclerotised. Ductus bursae short, close to corpus bursae strongly sclerotised, towards antrum dilating. Corpus bursae with strong anterior and posterior dilatations, constricted between; posterior half sclerotised, strongly ridged; anterior half membranous, plicate. Signum absent.
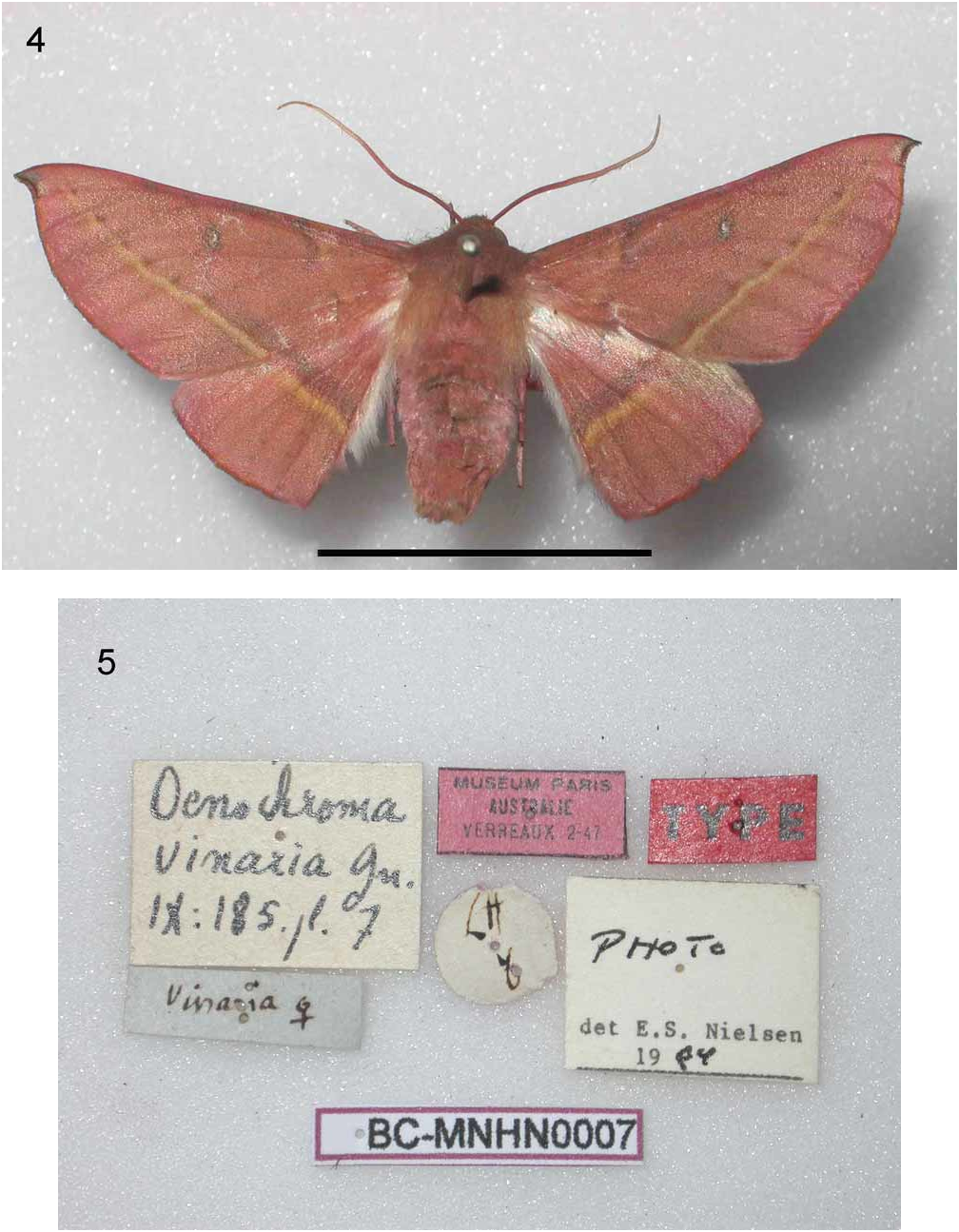
Morphological diagnosis (most congeners illustrated in BOLD (2008)): Oenochroma vinaria in habitus and external characters ( Figs. 4 View FIGURES 4, 5 , 6 View FIGURES 6, 7 ) very close to Oenochroma barcodificata and externally only distinguished, usually, by the straighter postmedian fascia of the forewing, often with a continuous dark proximal border. Dark suffusion of ground colour and blackish dotting of forewing costa usually reduced. Oenochroma pallida Warren, 1898 differs from both Oenochroma barcodificata and Oenochroma vinaria at once by the fore tibiae having an anterior apical hook (cf. Prout 1910, Turner 1932), and by the ochreous brown fringe of wing termen, the larger forewing cell spot on a paler ground colour, inner termen of hindwing underside with a narrow, dark spot; Oenochroma orthodesma Lower, 1894 has pale ochreous grey ground colour and ochreous fringe, an ochreous postmedian line edged anteriorly with pale yellow, the (ochreous) discal dots mostly wanting, hindwing at apex with pink suffusion and no spot on underside; Oenochroma decolorata Warren, 1896 , has grey forewings with fine brown irroration and purplish fringe, and a pale ferruginous postmedial fascia on both wings; Oenochroma phyllomorpha Lower, 1899 is of light brown ground colour, the forewing postmedial line is sinuate, fringe fuscous, and the cell spot lacking; Oenochroma cycnoptera Lower, 1894 has anterior tibiae with a strong apical hook (cf. Turner 1932), a very faintly ochreous postmedian line not reaching apex of forewing, fringe pale brownish, hindwings pale, without pattern and with whitish fringe; the New Caledonian Oenochroma unifasciata Holloway, 1979 , is broad-winged, without discal dots to the forewing.
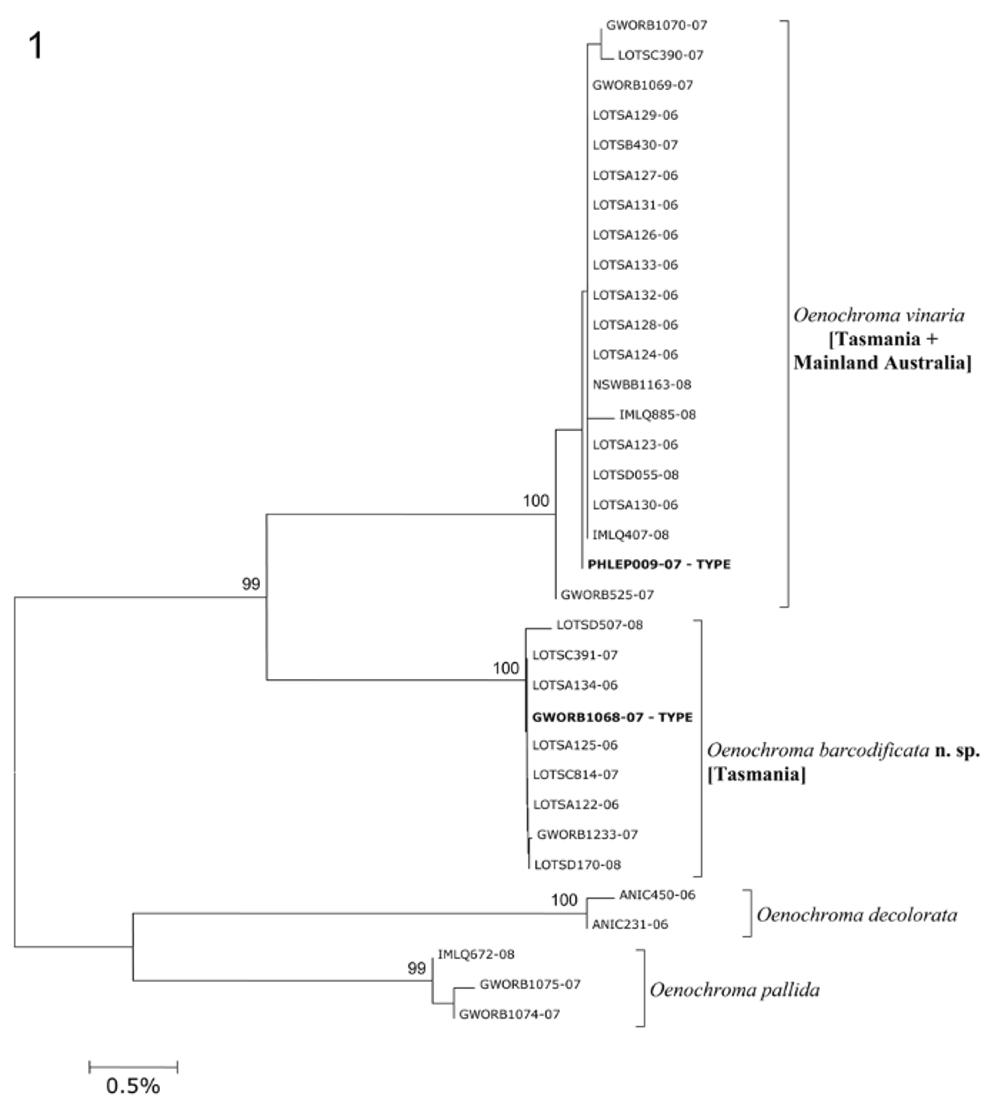
Molecular diagnosis (see Fig. 1 View FIGURE 1 and Table 2 View TABLE 2 ): DNA barcode analysis revealed a 3.34% K2P divergence between Oenochroma vinaria and O. barcodificata . Each species displays a very low mean intraspecific variation, with 0.05% (SE=0.03%, maximum distance of 0.33%) and 0.03% (SE=0.03%, maximum distance of 0.15%) for O. vinaria and O. barcodificata respectively and are thus unambiguously characterized by their DNA barcodes.
Larval hostplants. Reared on Grevillea sp. ( Proteaceae ).
Etymology. The species name refers to the barcoding campaign for Australia, and especially the fact that the new species could be distinguished by DNA barcoding without the need for dissecting (and thus damaging) an antique type specimen.
TABLE 2. Oenochroma vinaria species-group. Genetic distance calculations. A: intraspecific mean K2P divergences; B: interspecific mean K2P divergences. Standard error estimate(s) were obtained by a bootstrap procedure (1000 replicates).
| A | mean distance | standard error | maximal distance | sample size |
|---|---|---|---|---|
| O. vinaria | 0.05% | 0.03% | 0.33% | n=20 |
| O. barcodificata | 0.03% | 0.03% | 0.15% | n=9 |
| O. pallida | 0.00% | n=3 | ||
| O. decolorata | 0.15% | n=2 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Oenochrominae |
|
Genus |