Brenthia yangi, Liu, Tengteng, Wang, Shuxia & Li, Houhun, 2016
|
publication ID |
https://doi.org/10.11646/zootaxa.4079.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:642D5B0A-50DB-4805-8C86-199B735F3924 |
|
DOI |
https://doi.org/10.5281/zenodo.5628039 |
|
persistent identifier |
https://treatment.plazi.org/id/0386445B-FFBE-FF84-FF3B-FDF498C4997F |
|
treatment provided by |
Plazi |
|
scientific name |
Brenthia yangi |
| status |
sp. nov. |
Brenthia yangi sp. nov.
( Figures 1−22 View FIGURE 1 View FIGURE 2 View FIGURES 3 − 6 View FIGURES 7 − 11 View FIGURES 12 − 13 View FIGURES 14 − 19 View FIGURES 20 − 22 )
Brenthia View in CoL sp. Yang, 1977: 115.
Diagnosis. The new species resembles B. yaeyamae Arita, 1971 in forewing pattern, but can be separated by the male genitalia. Brenthia yangi sp. nov. has a juxta that is anchor-shaped at base and Y-shaped in distal 2/3, and a valva that has a distinct dorsal part and a ventral part, whereas B. yaeyamae has a rounded juxta and a strongly reduced valva without a dorsal part.
Adult ( Fig. 1 View FIGURE 1 a) with wingspan 7.0− 7.4 mm. Head greyish fuscous to dark fuscous, with purple-bronze gloss; face slightly tinged with greyish white; area dorsal to compound eyes silvery white; occiput with long lateral scales bending towards middle ( Fig. 1 View FIGURE 1 c–d). Antennae blackish fuscous dorsally, white ventrally. Labial palpus white, basal segment with a tuft of black mixed with white scales ventro-distally, second segment with a dark fuscous ring distally, third segment with similar rings basally and distally, blunt apically ( Fig. 1 View FIGURE 1 c–d). Thorax and tegula fuscous, with purple-bronze gloss. Legs white; foreleg with tibia black dorsally, with a black ring distally, tarsus with first segment in distal part and distal three segments black; middle and hind legs with tibiae having black rings before middle and at apex, tarsi with black rings at apex of first segment, with grey to black dots at middle of second segment dorsally, distal three segments black. Forewing greyish fuscous to blackish fuscous; a sinuate white or pale greyish-fuscous fascia at about basal 1/4, curved inwardly at posterior 1/3 and then straightly reaching dorsum near base, with several silvery scales set anteriorly; irregular white to pale greyish-fuscous stripes from before middle to terminal fascia; a reniform white ring beyond end of cell; an oblique outwards greyish stria before costal 3/4; terminal fascia black, its inner margin edged with greyish or pale greyish-fuscous line, with a violet dot placed near costa, its inner half interrupted by pale yellowish-fuscous scales to three black patches; termen with two white dots on anterior part, placed one above another, lower one larger, with three to four violet dots placed along posterior part, paralleled with a row of some six somewhat united violet dots near termen; cilia with basal 2/5 dark greyish fuscous, distal 3/5 dark grey. Hindwing blackish fuscous to black; an oblong white ring from costal 1/3 to lower angle of cell; an arc-shaped discontinuous white streak from costal 5/6 to dorsum before tornus, with a violet spot below costa; apex with a violet bar; cilia with basal 1/3 dark greyish fuscous, distal 2/3 blackish fuscous tinged with greyish white, forming two wedged white patterns posteriorly, apex white. Wing patterns similar on ventral surface ( Fig. 1 View FIGURE 1 b). Abdomen blackish fuscous dorsally, white with blackish-fuscous transverse streaks ventrally.
Venation ( Fig. 2 View FIGURE 2 ): Matches the generic characters defined by Diakonoff (1986).
Male genitalia ( Figs. 3−5 View FIGURES 3 − 6 ): Socius digitate, with two short hairs basally and about 10 longer hairs distally. Tegumen laterally sclerotized heavily, curved inwards triangularly. Tuba analis sclerotized ventrally, membranous dorsally. Vinculum a belt. Saccus triangular, pointed apically; apex connected to an almost heart-shaped sclerotized plate, which united with vinculum by membrane laterally, being free distally and blunt apically. Valva with dorsal part belt-like, its basal half narrow, bending ventrad, its distal half wider, irregular rectangular, densely covered with short and thick bristles, round apically; ventral part elongate triangular, broad at base, gradually narrowed to basal 2/3, distal 1/3 distinctly narrowed, clavate, bluntly pointed apically, ventral margin weakly sclerotized medially, sparsely haired. Anellus a weakly sclerotized tube with a dorso-distal process. Juxta anchor-shaped at base, Y-shaped in distal 2/3 ( Fig. 4 View FIGURES 3 − 6 b). Aedeagus ( Fig. 4 View FIGURES 3 − 6 c) tube-like, with a tubular process before ventro-apex, cornuti absent.
Female genitalia ( Fig. 6 View FIGURES 3 − 6 ): Papillae anales wide and short, with a row of thick hairs basally, with dense thin hairs distally. Posterior apophysis straight, not reaching the posterior margin of 7th tergite; anterior apophysis shorter than posterior apophysis, thicker. Ostium bursae sclerotized as a tube, protruded outside ventral surface; 7th sternite almost trapezoid, with posterior margin concave. Ductus bursae thin, membranous, almost as long as seventh segment. Corpus bursae membranous, oval, signum absent.
Last instar larva ( Fig. 20 View FIGURES 20 − 22 ): 4.0− 4.5 mm in length. Translucent, yellowish white when starving, yellowish green after feeding; pinacula fuscous. Head capsule light yellow, with dark brown spots.
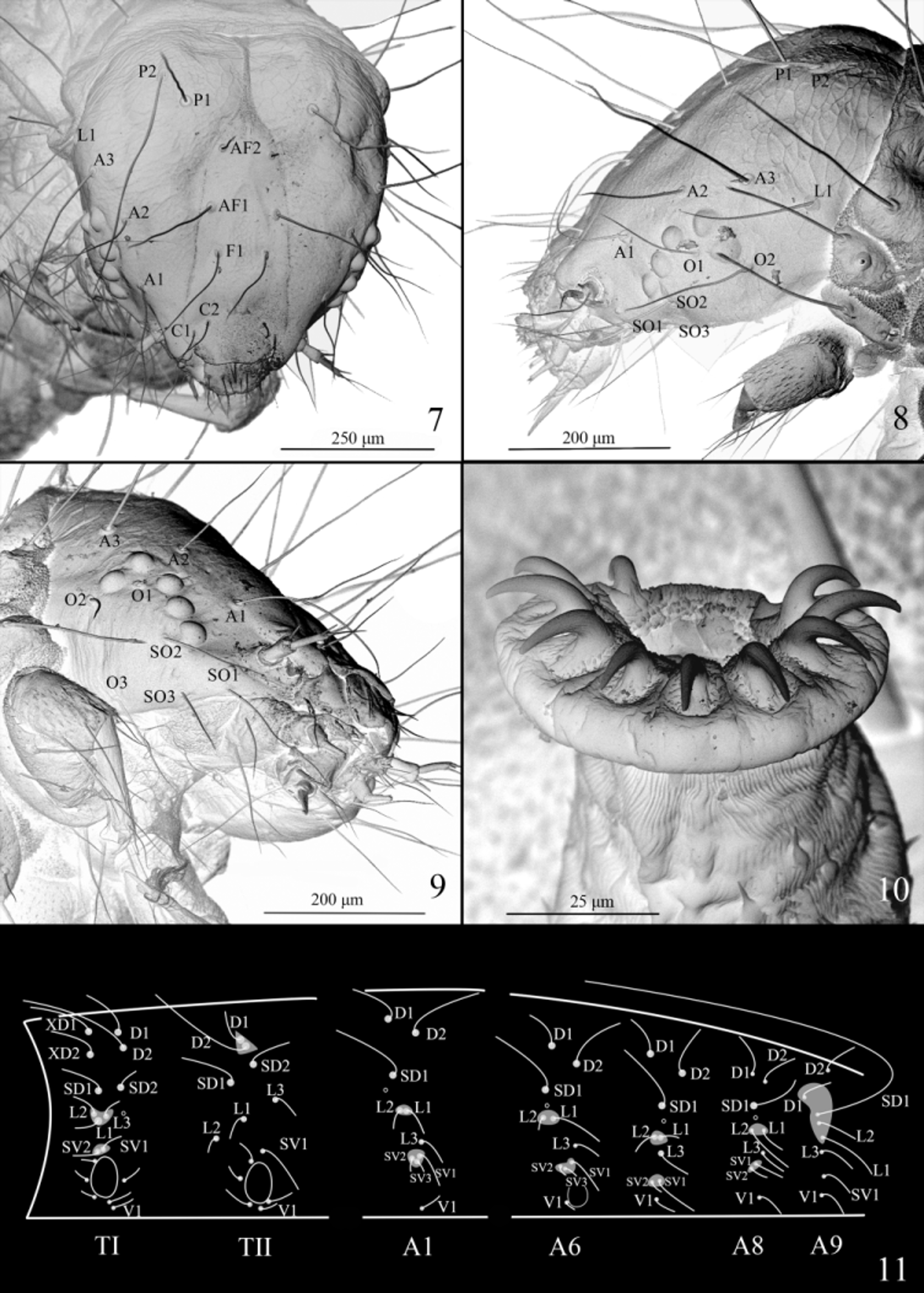
Head ( Figs. 7−9 View FIGURES 7 − 11 ). Five stemmata arranged in C shape, with stemma II slightly diverged, stemmata III −V nearly in a line. F1 before middle of frons; AF1 beyond middle of adfrontal area, longer than F1 and AF2, AF2 near apex of adfrontal area; A1 dorsal to stemma V, A2 dorsal to stemma II, A3 longest, posterolateral to A2, posterior to stemma II; P1, P2, and AF 2 in a line, P2 longer than P1; L1 posteroventral to A3; O 1 between stemmata I and III, O 2 posterior to stemma I, O 3 ventral to O 2; SO1 anteroventral to stemma V, ventral to antennal base, SO2 posterior to stemma V, SO3 ventral to stemma V, posterior to SO1.
Thorax ( Fig. 11 View FIGURES 7 − 11 ). TI with D2 posterior to and slightly dorsal to XD2; SD1 ventral to and slightly posterior to XD2, SD2 ventral to D2, posterior to and slightly dorsal to SD1; L1 ventral to middle of L2 and L3, spiracle posterior to L3; SV-group bisetose; L-group and SV-group each sharing its own pinaculum. TII with D1 and D2 sharing pinaculum, D2 longer than D1; SD1 anteroventral to D2, SD2 posteroventral to D2, posterodorsal to SD1; L1 anteroventral to L3, posterodorsal to L2; SV-group unisetose. TIII similar to TII.
Abdomen ( Fig. 11 View FIGURES 7 − 11 ). A1 to A8 with D1 anterodorsal to D2, L1 posterior to L2 and sharing pinaculum with L2, SV-group sharing pinaculum, having three setae on A1−6, bisetose on A7−8; A9 with D1 anteroventral to D2, SD1, L2, L1, L3, and SV1 nearly in a line, D1, SD1, L2, and L1 sharing pinaculum, SD1 extremely long. Prolegs with 9−10 crochets, uniserial and uniordinal, arranged in mesal pennelipse ( Fig. 10 View FIGURES 7 − 11 ).
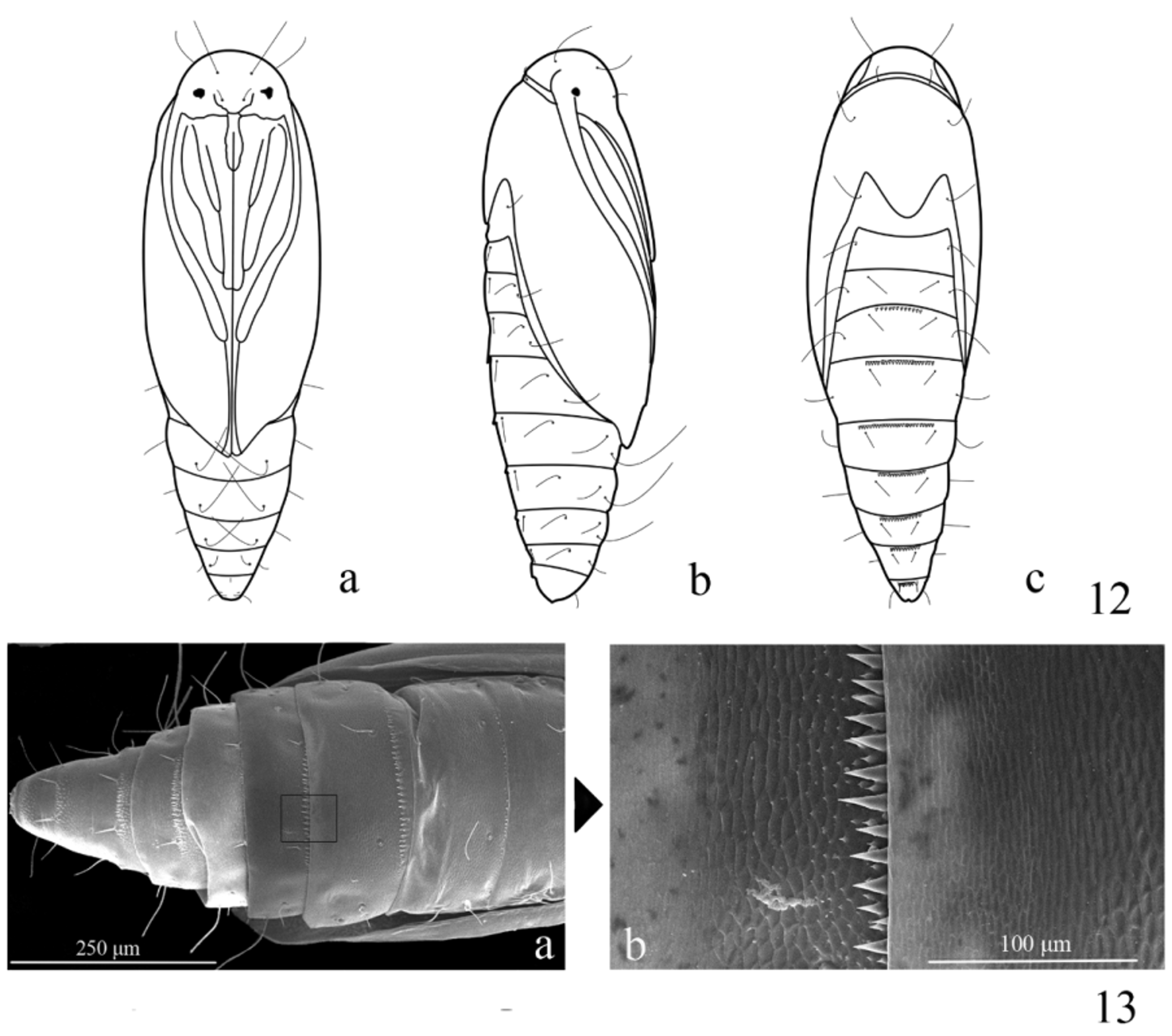
Pupa ( Fig. 12 View FIGURES 12 − 13 ): 3.25 mm in length (n = 3). A3−9 each with a row of dense dorsal spines beyond anterior margin, spines on A3 and A9 weaker ( Fig. 13 View FIGURES 12 − 13 ); A5−7 with ventral setae long and curved forward ( Fig. 12 View FIGURES 12 − 13 ). The curved setae on A5−7 can hook with the cocoon to stabilize the pupa at adult eclosion ( Fig. 14 View FIGURES 14 − 19 ), otherwise the pupa would fall from the cocoon and fail to emerge. Cocoon white, rhombic, consisting of two silken walls and two pieces of oval silken layers between them ( Figs. 15−16 View FIGURES 14 − 19 ); longitudinal ends connected with silken web that attaches onto leaf ( Fig. 17 View FIGURES 14 − 19 ).
Type Material: Holotype, ♂, CHINA: Tianjin: Mt. Baxian National Nature Reserves ( 40°11'N, 117°32'E), Ji County, 300−600 m, larva collected on Grewia biloba on 6.ix.2013, emerged on 8.iv.2014 (indoors), leg. Tengteng Liu. Paratypes: 1 ♀, same data as holotype, except dated viii.2013, pupa kept indoors, emerged on 31.xii.2013; 4 ♂, 6 ♀, same data as holotype, except emerged on 28, 31.iii.2014, 1, 5, 8.iv.2014, iv.2014; 2 ♂, larvae collected on 24.vi.2014, emerged on 12.vii.2014, other same data as holotype; 11 ♂, 17 ♀, same data as holotype, except dated 20, 28−30.vi, 7−8.viii.2013, collected by net; 1 ♀, 300 m, pupa collected on 7.viii.2013, emerged on 8.viii.2013, other same data as holotype; 1 ♂, 26.vi.2014, by net, other same data as holotype; 1 ♂, 270 m, 21.v.2009, adult collected by net, leg Bingbing Hu, other same data as holotype; 1 ♂, 1 ♀, pupae collected on 22.vii.2012, emerged on 1.viii.2012, leg. Shurong Liu, other same data as holotype; 1 ♂, Mt. Jiulong ( 40°08'N, 117°31'E), Ji County, 130 m, 9.vi.2004, by light trap, leg. Houhun Li; Shanxi Province: 3 ♂, 4 ♀, Mang River Nature Reserves ( 35°15'N, 112°27'E), Yangcheng, 580 m, 1.viii.2013, by light trap, leg. Shulian Hao et Mingjing Li; Henan Province: 7 ♂, 2 ♀, Mt. Song ( 34°28'N, 112°56'E), Dengfeng, 800 m, 9.vi.2000, by light trap, leg. Haili Yu; Shandong Province: 1 ♂, Zhongshan Temple ( 35°47'N, 118°10'E), Mt. Yimeng, Linyi City, 28.vii.2009, by light trap, leg. Qing Jin; Beijing: 1 ♀, Mt. Xiang ( 39°59'N, 116°11'E), 26.vi.1964, leg. Fasheng Li ( CAU); 1 ♀, Mt. Xiang, vi.1957, leg. Chinese Academy of Sciences.
Additional material. Shanxi Province: 1 ex. (without abdomen and hindwings), Mang River Nature Reserves ( 35°15'N, 112°27'E), Yangcheng, 580 m, 1.viii.2013, by light trap, leg. Shulian Hao et Mingjing Li; Henan Province: 1 ♀ (without abdomen), Mt. Song ( 34°28'N, 112°56'E), Dengfeng, 800 m, 9.vi.2000, by light trap, leg. Haili Yu; Henan Province: 1 ♂ (without abdomen), Mt. Wangwu ( 35°10'N, 112°17'E), Jiyuan, 700 m, 5.vi.2000, leg. Haili Yu.
Distribution. China (Beijing, Henan, Shandong, Shanxi, Tianjin).
Etymology. The specific name is dedicated to the late Professor Jikun Yang, China Agricultural University, Beijing, for his first record of this species.
Biology. Brenthia yangi sp. nov. feeds on leaves of Grewia biloba G. Don (Malvaceae) and its variety parviflora Hand. -Mazz ( Fig. 18 View FIGURES 14 − 19 ). Brenthia paranympha Meyrick, 1912 , recorded from Assam, India, was the first species of Brenthia known to feed on Grewia plants ( Robinson et al. 2010); B. yangi sp. nov. is the second species feeding on this plant genus.
The larva feeds on the underside of a leaf. The last instar larva tends to pupate on the underside of a host plant leaf, possibly to hide from predators, but perhaps also because of the microstructure of the leaf. The upper surface of the leaf of G. biloba is smooth or sparsely pubescent, while the lower surface is densely pubescent with stellate hairs ( Fig. 19 View FIGURES 14 − 19 ), which thus facilitates fixing of the silken web and provides better anchoring of the cocoon ( Fig. 17 View FIGURES 14 − 19 ). After pupation, the exuvium of the last instar larva is kept in one of the longitudinal ends, or is pushed outside the cocoon; the pupa protrudes from the opposite end at emergence ( Fig. 15 View FIGURES 14 − 19 ). Emergence usually occurred in day time in the laboratory. Adults occurred from late May to August, and larvae occurred from late June to September at the type locality. This species overwinters as a pupa.
Larvae of different stages set escape holes differently. The position selection of escape holes was studied by statistical analysis. The results are as follows.
a. Escape holes chewed by all instar larvae are traceable on leaves after the larvae have gone. Escape holes are set along the margin of the feeding area ( Fig. 20 View FIGURES 20 − 22 ). Each escape hole is round, and closely approximated to a vein where a silken pillar containing numerous fecal pellets is tightly connected ( Fig. 21 View FIGURES 20 − 22 ); the larger the diameter of the escape hole is, the thicker the relevant silken pillar. When threatened, the larva has to move the longest distance to reach the escape hole ( Fig. 20 View FIGURES 20 − 22 ). We observed that a larva would move underside and create an escape hole as soon as it was transferred to the upper surface of a new leaf.
b. The diameters of the escape holes of each instar larva are shown in Table 1.
c. The number of differently positioned escape holes of I −II and III −V instar larvae are shown in Table 2 View TABLE 2 , and the accumulative results of escape holes are shown in the leaf diagram ( Fig. 22 View FIGURES 20 − 22 ). Larvae of I–II instar have no significant preference for the position of escape holes ( t = −1.957, P = 0.060> 0.05), but 38.3% (67 / (67+108)) of escape holes are set along main veins. III −V instar larvae significantly preferred to set escape holes along main veins ( t = 6.299, P <0.001).
d. The number of escape holes in the corner of two veins and elsewhere of III −V instar larvae are shown in Table 3 View TABLE 3 . The results indicated that the corner of two veins was not always the most preferred position for III −V instar larvae, despite the fact that the corners of veins were not all taken.
** Indicating significant differences ( t = 6.299, P <0.001) Larvae of III −V instar preferred significantly to set escape holes along the main veins; I −II instar larvae showed no specific preference for the main veins, but their escape holes were usually set close to veins. The silken pillar, coexisting with the escape hole, was always fixed to a vein, and for this reason the escape hole was usually set close to a vein. Larvae of III −V instar probably need main veins to fix their thicker silken pillars, whereas for I −II instar larvae, the main veins, lateral veins, and/or even micro-veins are probably suitable enough to fix their thinner silken pillars. Consequently, larvae of different stages preferred different positions of escape holes, and the size of the silken pillar determined the positions of escape holes statistically in the III −V instar larvae. Rota (2008) suggested that fecal stalactites might serve to suspend the webbing above the larva, which is similar in the present case. The silken pillar probably serves to support the webbing that covers the feeding area, and a thicker silken pillar probably can support a larger feeding area for an older larva.
Williams (1951) reported that B. leptocosma preferred to set escape holes in the corner of two veins, which is not consistent with the behavior in B. yangi sp. nov. Arita (1971) reported a similar defense strategy in L. japonica . Such a defense strategy is probably practiced by both Brenthia and Litobrenthia , and thus may represent a behavioral synapomorphy for the Brenthiinae .
TABLE 2. Number of differently positioned escape holes by I − II and III − V instar larvae.
| Leaf code 0 1 | 0 2 | 0 3 | 0 4 | 0 5 | 0 6 | 0 7 | 0 8 | 0 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instars Along main 4 I −II vein | 4 | 4 | 1 | 6 | 3 | 5 | 5 | 3 | 10 | 5 | 1 | 0 | 3 | 4 | 9 | 67 |
| Elsewhere 1 | 16 | 3 | 11 | 5 | 6 | 7 | 7 | 6 | 14 | 11 | 1 | 10 | 4 | 2 | 4 | 108 |
| Instars Along main 8 III −V vein | 1 | 10 | 6 | 8 | 7 | 4 | 6 | 8 | 12 | 8 | 8 | 7 | 15 | 5 | 9 | 122** |
| Elsewhere 0 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 2 | 6 | 6 | 0 | 1 | 1 | 4 | 2 | 28 |
TABLE 3. Number of escape holes in a corner of veins and elsewhere of III − V instar larvae.
| Corner of two veins | Elsewhere | |
|---|---|---|
| Number of escape holes | 33 | 117 |
| Discussion |
| CAU |
China Agricultural University |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Brenthiinae |
|
Genus |
Brenthia yangi
| Liu, Tengteng, Wang, Shuxia & Li, Houhun 2016 |
Brenthia
| Yang 1977: 115 |