Parena Motschulsky, 1860
|
publication ID |
https://doi.org/10.11646/zootaxa.5286.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:F9834684-24D3-4795-B5EB-77B451DF856D |
|
DOI |
https://doi.org/10.5281/zenodo.7963691 |
|
persistent identifier |
https://treatment.plazi.org/id/03877623-6240-FFFB-2DEF-B18DFA7A5DB2 |
|
treatment provided by |
Plazi |
|
scientific name |
Parena Motschulsky, 1860 |
| status |
|
Genus Parena Motschulsky, 1860 View in CoL View at ENA
Type-species: Parena bicolor Motschulsky View in CoL , fixed by monotypy.
Parena Motschulsky, 1860: 31 View in CoL ; Chaudoir, 1877: 207; Peringuey, 1896: 242, (misspelled as Pazena View in CoL ); Andrewes, 1928: 16; Jeannel, 1949: 971; Jedlička, 1963: 439; Habu, 1967: 149; Habu, 1982: 109; Shpeley, 1986: 269; Xie & Yu, 1993: 185; Kirschenhofer, 1994: 1022; Kirschenhofer, 2006: 87.
Phloeodromius Macleay, 1871: 85 View in CoL , Type-species: Phloeodromius piceus Macleay View in CoL , by monotypy; Sloane, 1898: 493; Andrewes, 1919: 483; Andrewes, 1921: 179; Andrewes, 1928: 16 (synonymized with Parena Motschulsky View in CoL ).
Umgenia Peringuey, 1898: 324 View in CoL , Type-species: Umgenia formidulosa Peringuey View in CoL , by monotypy; Peringuey, 1904: 296 (synonymized with Metallica Chaudoir View in CoL ); Basilewsky, 1961: 213 (synonymized with Parena Motschulsky View in CoL ).
Prymira Fairmaire, 1899: 76 View in CoL , Type-species: Prymira stigmatica Fairmaire View in CoL , by monotypy; Alluaud, 1917: 87 (subgen. of Crossoglossa View in CoL ); Csiki, 1932: 1455 (synonymized with Parena Motschulsky View in CoL ).
Euprymira Fairmaire, 1901: 122 View in CoL . redundant replacement name for Prymire Fairmaire.
Recognition.
In addition to the characters states of subtribe Metallicina ( Shpeley, 1986: 268) , adults of the genus Parena can be recognized by: Head and pronotum yellowish brown to piceous, not metallic; elytra color various, unicolor or with pattern, metallic or not; antennomeres 5–11 with sensory pits on ventral and dorsal surfaces; elytral interval 9 less than half width of normal intervals; each claw with six to ten long pectinations; male mesotarsi with biseriate adhesive setae ventrally. Endophallus of male genitalia with a well chitinized primary sclerite; without flagellum or with flagellum much shorter than aedeagus (compared to Euproctinus ); without a group of spines (compared to Metallica and Pachycallida ). Gonocoxite II of female ovipositor subquadrate or dichotomous, with four to seven ensiform setae, without membranous apex.
Compared with other Lebiini genera showing similar appearances (e.g., Calleida , Paraphaea , Peliocypas ), Parena can be recognized by the combination of following external features: mandibles strongly widened; mentum without median tooth; apex of glossal sclerite with four or more apical setae; terminal palpomeres fusiform; cleaning spur close to inner margin of protibia; tarsomeres 4 bilobed; claws strongly pectinate.
Morphology.
General features: Members of genus Parena are median-sized truncatipennes ground beetles, body length between 6.3–11.7 mm; body form sub-depressed, slightly widened at the apical third of elytra.
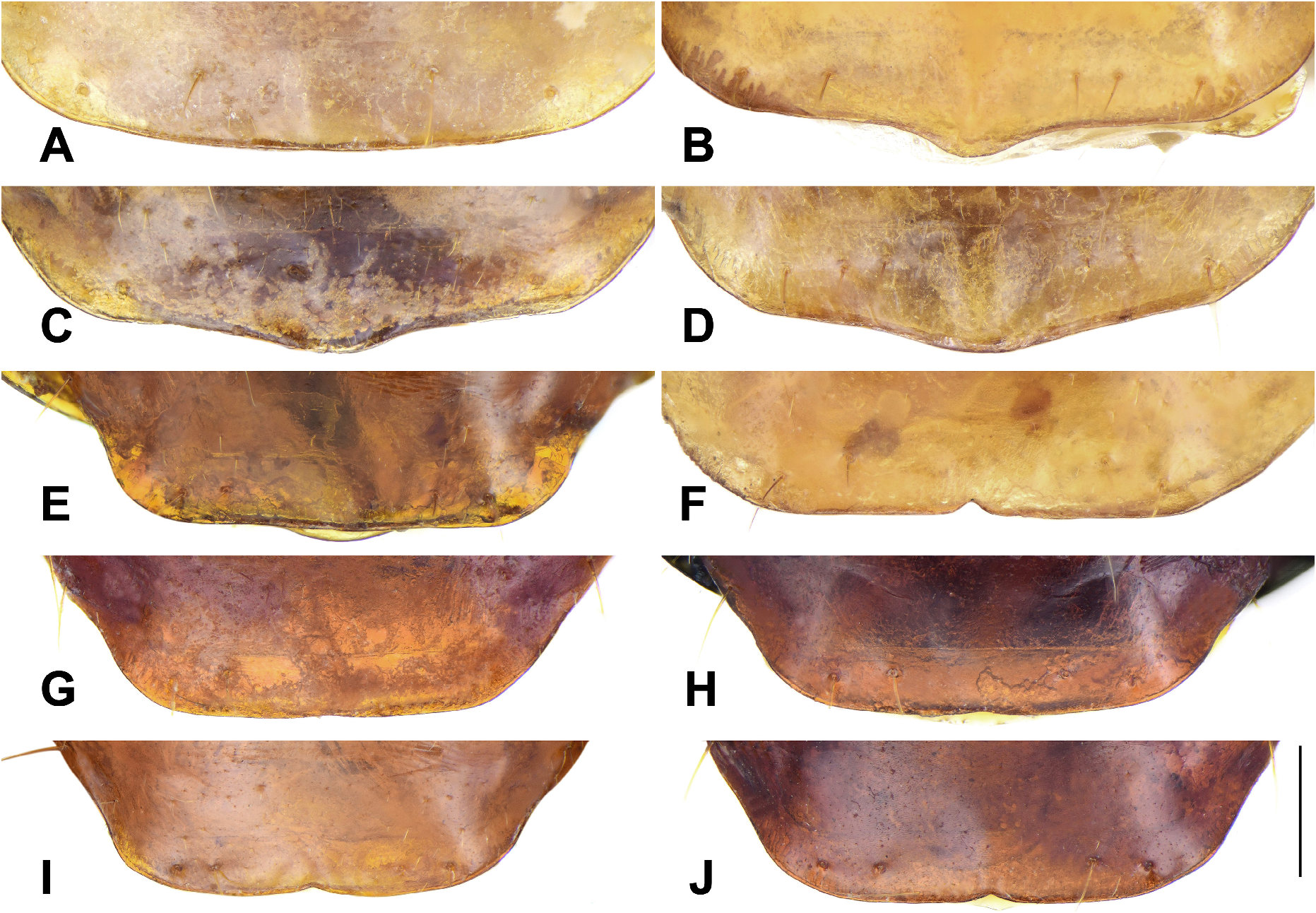
Color: The head uniformly reddish brown to piceous in most species. Frons with a triangular red patch in some dark species of subgenera Parena and Bothynoptera . Clypeus same color as frons or paler (in some dark species). Mouthparts slightly paler than the head in most species, with terminal palpomeres gradually lightened to apex. Antennomere 1 yellowish or reddish brown, slightly paler than head in most species. Antennomeres 2–4 same color as antennomere 1 in most species, but nearly black in some species of the P. testacea species group. Antennomeres 5–11, and also the apical half of antennomere 4 darkened in the subgenus Crossoglossa and part of the subgenus Parena , but same color as basal antennomeres in the subgenus Bothynoptera and part of the subgenus Parena . Pronotum with disc same color as the head, usually without a well-defined pattern, but with a pair of black stripes in P. emarginata sp. n.; lateral explanations somewhat lighter than disc. Elytral colors varied: uniformly brown or metallic, with diverse dark or light patterns, or with black or metallic lateral stripes. For some of those patterned species, the style of elytra pattern is stable and has importance in species determination; but in other cases, the elytra patterns present dimorphism (e.g., P. africana ) or pleomorphism (e.g., P. fasciata ), or are varied individually (e.g., P. nigrolineata ) or in different geographical populations (e.g., P. malaisei ). The entire venter same color as the pronotum in most species, with the exceptions of P. sciakyi sp. n. and P. heteronycha sp. n., which have the metasternum much darker than other parts. Major parts of the legs are usually in the same color as the pronotum. Femora are unicolor in most species, but those of P. picipes sp. n. are black at the apical half. Tibiae are usually the same color as the apical half of femora, with the only exception that the tibiae of P. cruralis are black in contrast to the pale yellow femora. Tarsomeres are the same color as the femora and tibiae for most species, but slightly lighter in some dark species (e.g., P. obscura ), or distinctly darkened in all members of the P. testacea group and some of these African species.
Microsculpture: Head and pronotum without microsculpture. Labrum with distinct isodiametric microsculpture. Elytra without microsculpture or with shallow isodiametric microsculpture (no species with transverse microsculpture). The presence of elytral microsculpture has no sexual dimorphism but is sometimes variable within species (e.g., P. tripunctata ).
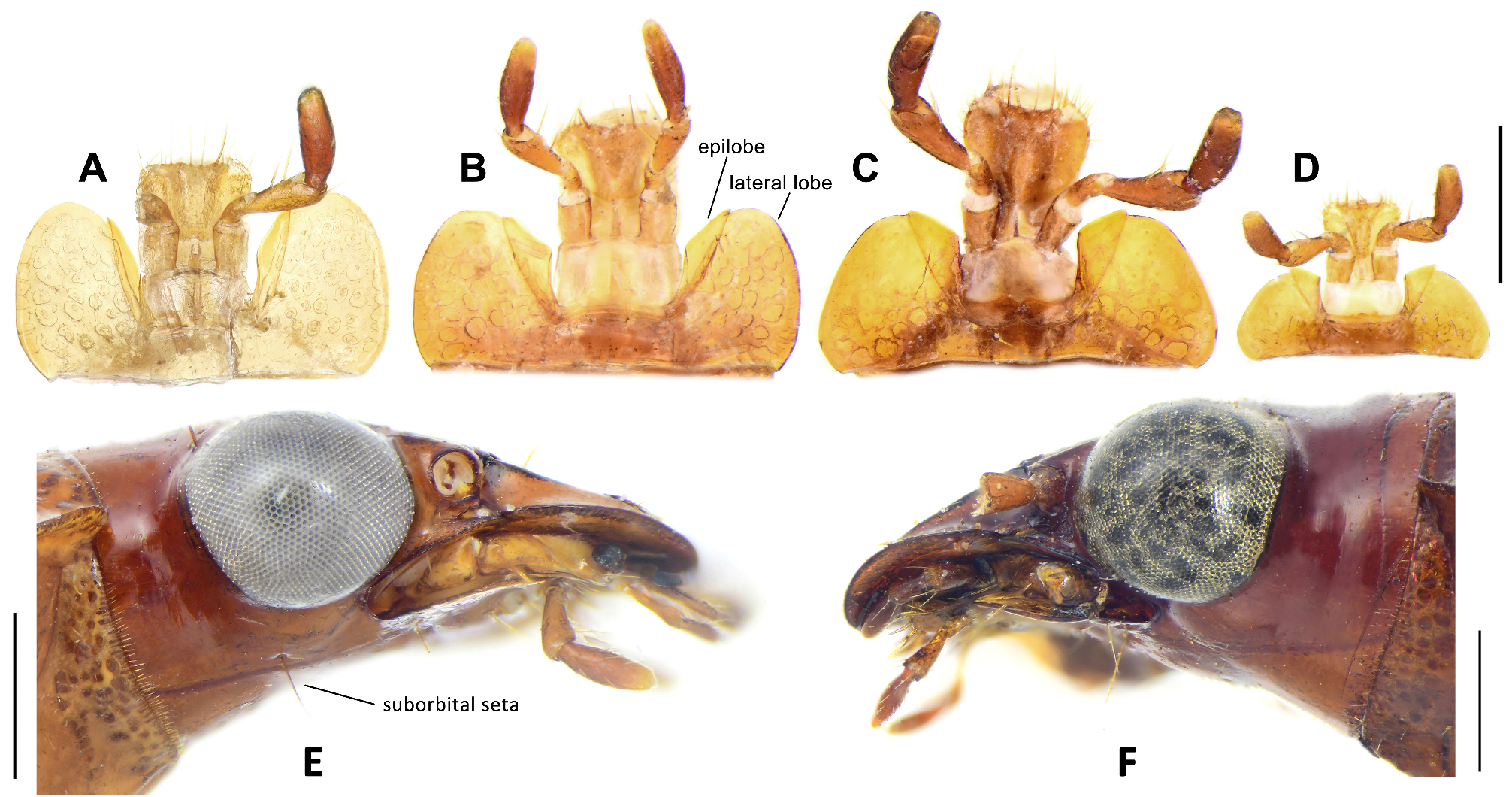
Head: Vertex shallowly swollen, with surface smooth or finely punctate. Dorsal surface of the head without accessory setae, but with sparse short setae behind the vertex in some species (e.g., P. dorsigera ). Frons with a Vshaped depression in the middle, shallow in some species but always recognizable. Frontal furrows shallow and wide, terminated before midpoint of the eyes. Frontoclypeal suture distinct in most species, but partly fused at the middle in some species. Clypeus subquadrate and bisetose. Eyes very large, hemispheric, and strongly convex. Tempora abruptly narrowed, very short with length approximately one-eighth diameter of eye in most species, but much longer in the P. tesari group (one-third to half diameter of eye). Head behind the tempora constricted, parallel-sided, forming the neck-constriction. The sum of the length of the neck-constriction and tempora (namely the distance between the posterior margins of eyes and pronotal anterior angles) is approximately one-third the diameter of the eye in most species, but more than two-thirds of the diameter of the eye in the P. tesari group. Postgenae with a pair of long suborbital setae close to the gular sutures in all species of subgenera Crossoglossa and Bothynoptera ( Fig. 3E View FIGURE 3 ). Suborbital setae are similar length to the supraorbital setae, but are short (less than half length of the supraorbital setae) in P. heteronycha sp. n. In the subgenus Parena , the suborbital setae absent ( Fig. 3F View FIGURE 3 ) for most species, but present in species of the P. bicolor and P. stigmatica groups. In a few individuals of the P. dorsigera group, there are two suborbital setae on each side.
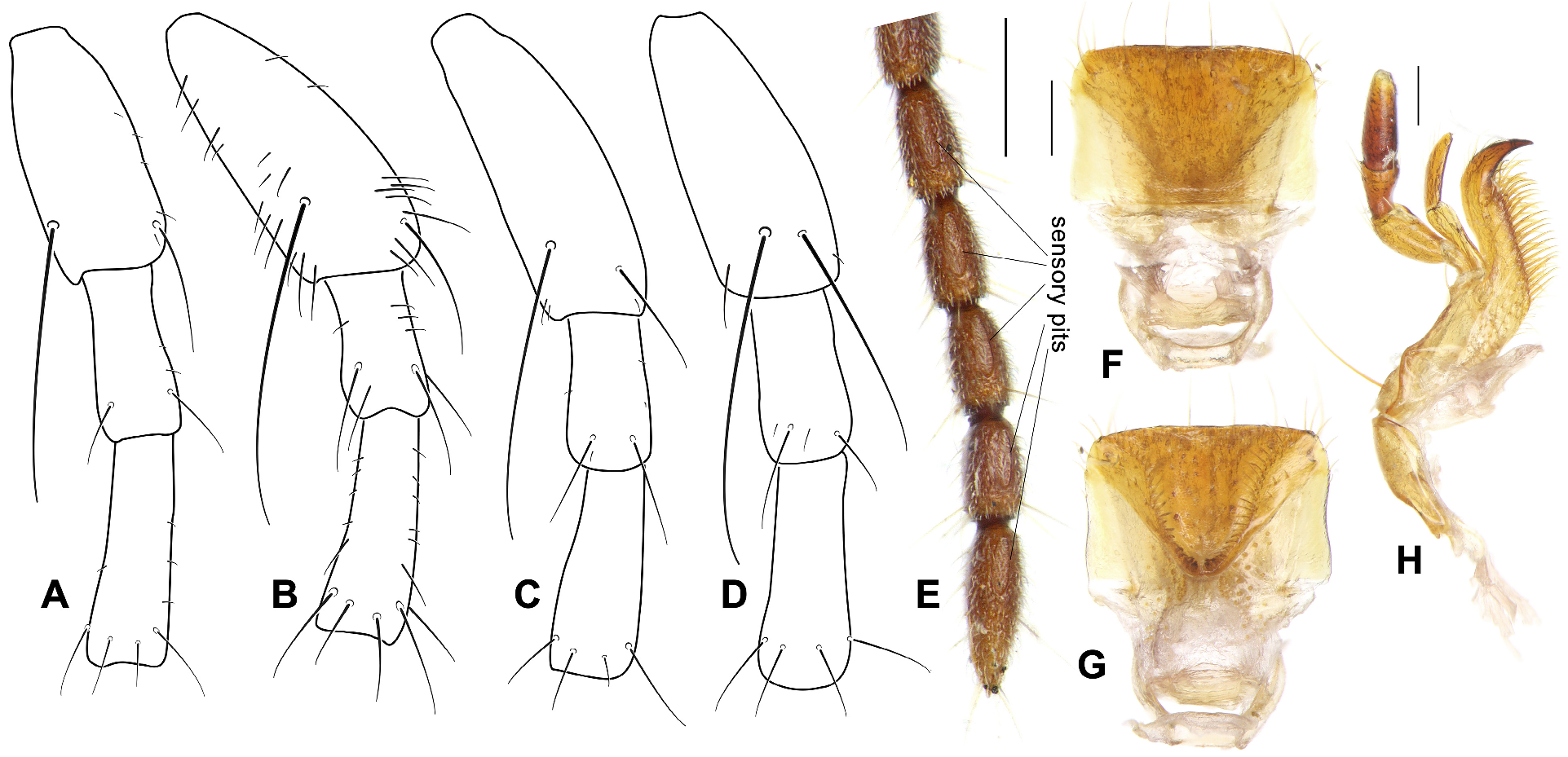
Antennae: Similar to other arboreal groups of Lebiini , members of Parena have relatively short antennae, barely extended to the base of pronotum in the subgenera Crossoglossa and Parena , extended about one antennomere length beyond the elytral base in the subgenus Bothynoptera . Antennomere 1 thick and arcuate, with a depressed posterior surface (accommodating the convex eyes), with dorsal-posterior margin distinctly ridged and ventral-posterior margin indistinctly ridged. Antennomere 1 with two primary setae near the apex, with the ventral one less than half length of the dorsal one ( Figs 1A, 1B, 1C View FIGURE 1 ) in most species, but only slightly shorter in the P. scutata group ( Fig. 1D View FIGURE 1 ). Antennomere 2 has two primary setae of similar length near the apex. Antennomere 3 has five or six primary setae forming the apical ring. The accessory setae on antennomeres 1–3 are absent or represented only by sparse, very short and fine ones in the subgenera Crossoglossa and Parena ( Figs 1A, 1C, 1D View FIGURE 1 ). Accessory setae are more abundant and longer in the subgenus Bothynoptera , with one or two of them on the antennomere 2 of similar length to the primary ones ( Fig. 1B View FIGURE 1 ). The apical half of antennomere 4 and all of the following more distal antennomeres are pubescent. Sensory pits are on both dorsal and ventral surfaces of antennomeres 5–11 ( Fig. 1E View FIGURE 1 ).
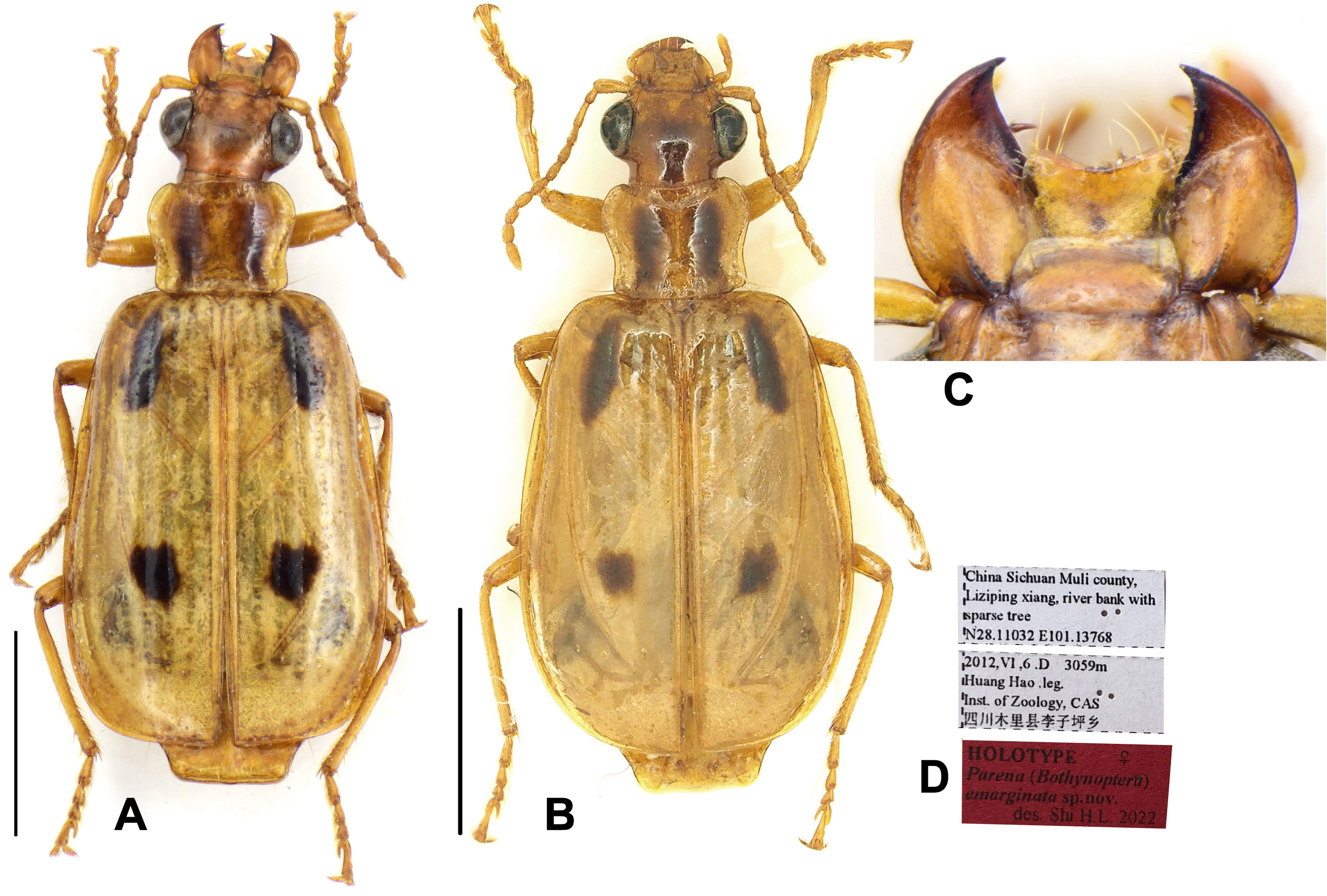
Mouthparts: Labrum ( Figs 1F, 1G View FIGURE 1 ) nearly quadrate or very slightly dilated towards the apex, length about two-thirds of width. with six apical setae almost evenly arranged. Apical margin nearly straight in most species ( Fig. 1F View FIGURE 1 ) or slightly convex, very weakly emarginate in several species of the P. tripunctata group and distinctly emarginate in P. emarginata sp. n. ( Fig. 46C View FIGURE 46 ).
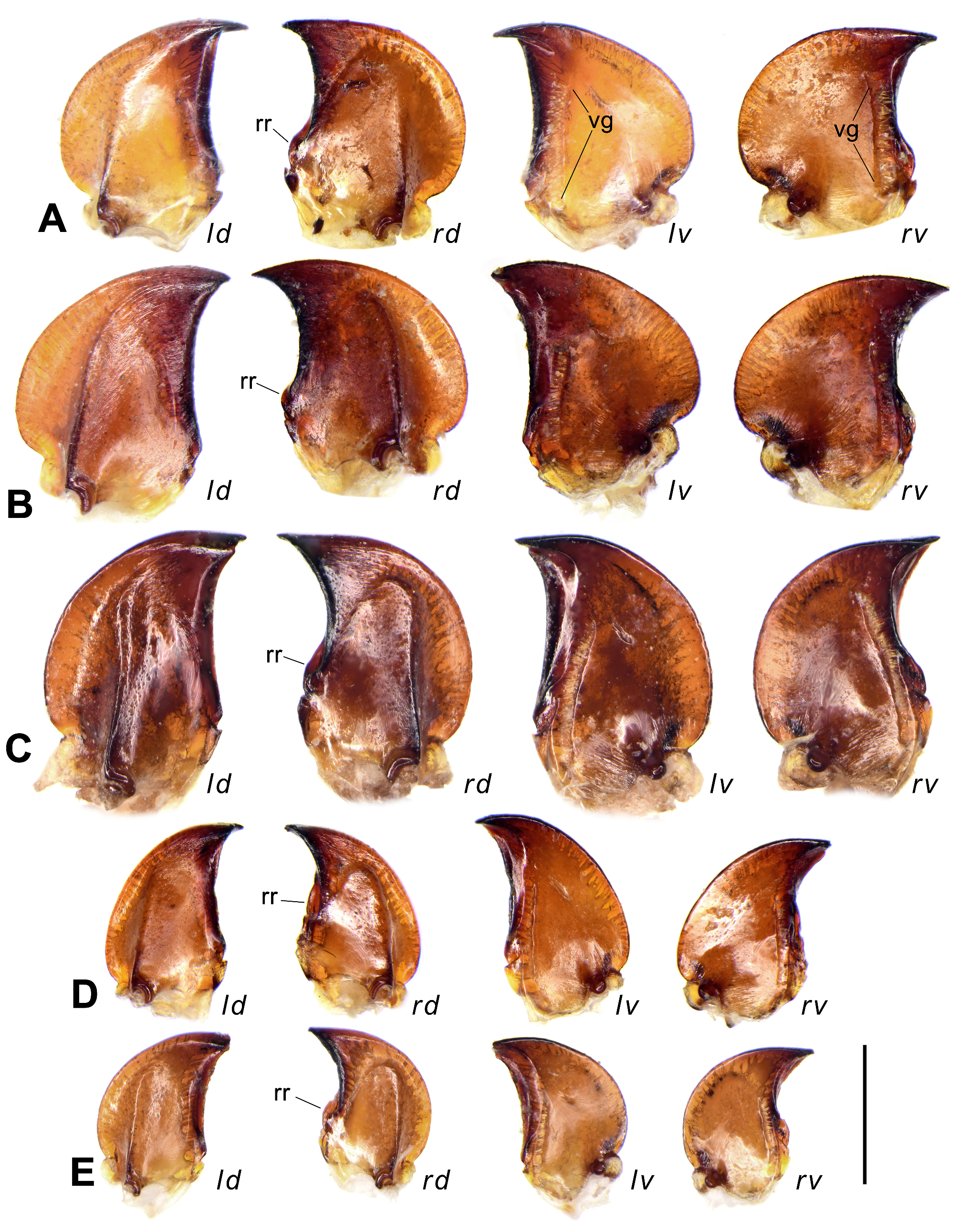
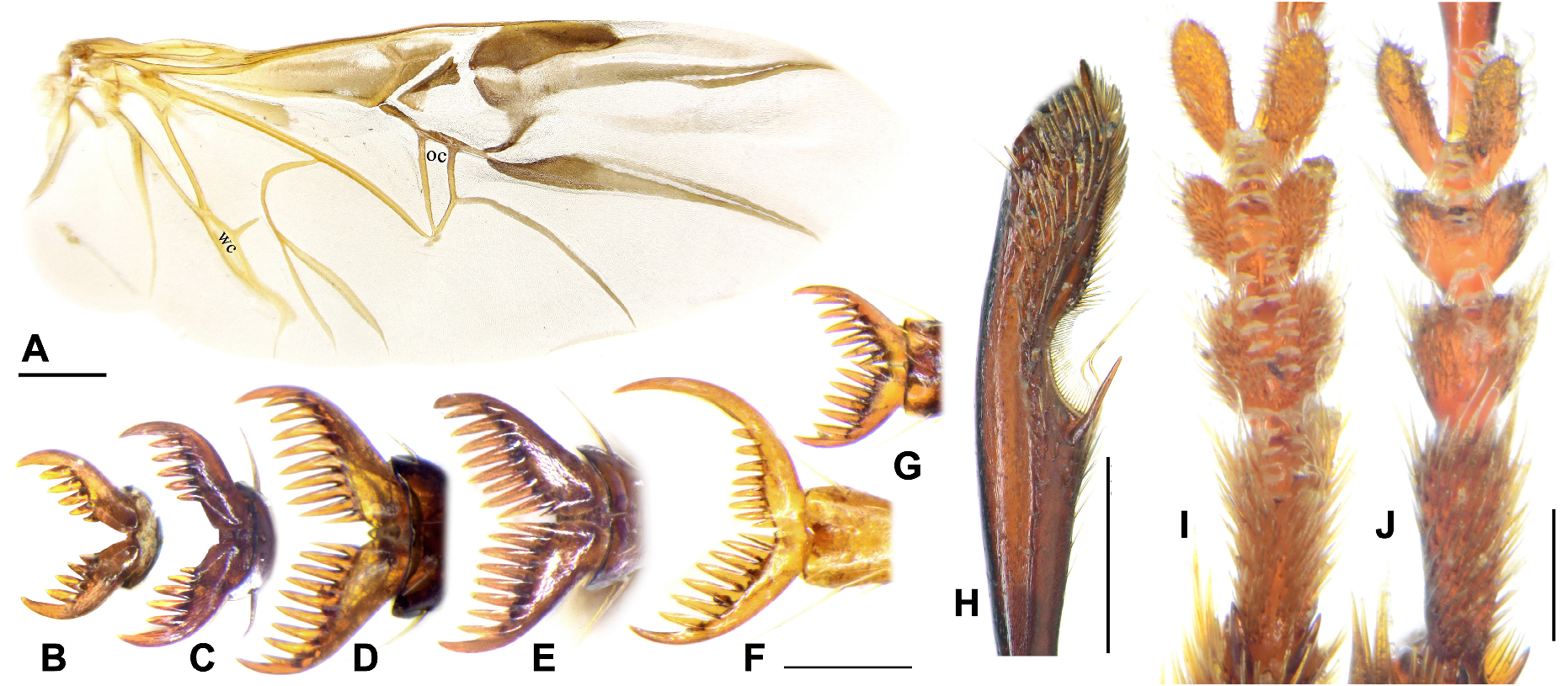
Mandibles widened, with arcuate external edges and strongly extended mandibular scrobes, conspicuously widened in subgenera Crossoglossa and Parena , with each roughly semicircular ( Figs 2A, 2B View FIGURE 2 ), and less widened in subgenus Bothynoptera , with each rounded-triangular ( Figs 2C, 2D, 2E View FIGURE 2 ). Mandibular apices very shortly hooked and suddenly turned inward, but slightly more elongate and less turned inward in P. heteronycha sp. n. ( Fig. 26C View FIGURE 26 ). Left mandible with inner edge of the apical hook formed by the shortened terebral ridge and the cutting edge formed by the retinacular ridge. Right mandible with the cutting edge formed by the terebral ridge. Retinacular ridge of right mandible reduced to a retinacular tooth (rr in Figs 2A, 2B View FIGURE 2 ) in subgenera Crossoglossa and Parena , but present as a shortened ridge in subgenus Bothynoptera . In most species of Bothynoptera (rr in Figs 2C, 2E View FIGURE 2 ), its length is about one-third of the terebral ridge in ventral view, but more than half of the terebral ridge in P. heteronycha sp. n. ( Fig. 26C View FIGURE 26 ) and P. taiwana (rr in Fig. 2D View FIGURE 2 ). Ventral grooves present, more than two-thirds length of the mandibular mesal edges.
Maxillae ( Fig. 1H View FIGURE 1 ): apex of lacinia strongly hooked, with a smooth inner curved surface. Terminal maxillary palpomeres fusiform in both sexes, glabrous and longer than the penultimate palpomere. Penultimate and antepenultimate palpomeres with several short setae near apex.
Mentum varied in shape, without a median tooth, with a pair of setae in subgenera Bothynoptera and Parena (very short in the P. nigrolineata and P. latecincta groups) and without such setae in Crossoglossa . In subgenus Crossoglossa ( Fig. 3A View FIGURE 3 ), lateral lobes extremely large, with inner margins nearly straight and outer margins fully arcuate; apices of lateral lobes widely rounded and extended well beyond the very narrow epilobes. In subgenera Parena and Bothynoptera ( Figs 3B, 3C, 3D View FIGURE 3 ), lateral lobes relatively short, with inner margins more or less oblique and outer margins nearly straight or arcuate, and the epilobes relatively wide; apices of lateral lobes narrowly rounded or slightly angulate, only slightly extended beyond the epilobes in subgenus Parena ( Fig. 3B View FIGURE 3 ), but the epilobes extended beyond the lateral lobes ( Figs 3C, 3D View FIGURE 3 ) in subgenus Bothynoptera . Glossal sclerite apically truncate, with two to four pairs of setae, the paramedian pair longer than the others. Paraglossae fused to the glossal sclerite, with apices membranous and setose. Terminal labial palpomeres fusiform or subcylindrical, similar in both sexes. Terminal and penultimate labial palpomeres shortly setose, the penultimate palpomere with two long setae on the inner margin and one on the dorsal apex.
Submentum with two setae on each side in most species, with the outer one much shorter than the inner one. However, there is only one long seta on each side of submentum in P. heteronycha sp. n.
Pronotum: Pronotal shapes are varied among species: nearly rectangular to distinctly transverse. The pronotum of the subgenus Crossoglossa is often wider (PW/PL = 1.50–1.82, generally greater than 1.60) than in other two subgenera, and that of the subgenus Bothynoptera is often narrower (PW/PL = 1.19–1.43, generally less than 1.35). The pronotum is much wider than the head (PW/HW = 1.05–1.26, generally greater than 1.10) in the subgenus Crossoglossa , narrower or barely wider than the head (PW/HW = 0.85–1.13, generally less than 1.10) in the other two subgenera. Anterior margin nearly straight or weakly emarginate at middle, without distinct apical angles. Posterior margin nearly straight at the middle, and more or less oblique laterally. Maximum width of pronotum near the anterior third, but in some species of subgenus Bothynoptera (e.g., P. heteronycha sp. n.), distance between posterior angles is almost equal to the maximum width. Lateral margins more or less sinuate before posterior angles in most species, with two marginal setae, at the maximum width point and the posterior angle, respectively. Posterior angles narrowly rounded or faintly angulate at an obtuse or rectangular angle in most species, but not denticulate. The lateral explanations more or less widened. Basal foveae shallow and wide, without distinct limits. Disc moderately convex, glabrous or finely and sparsely punctate, sometimes faintly wrinkled. Median line fine, slightly deepened anteriorly and posteriorly in some species.
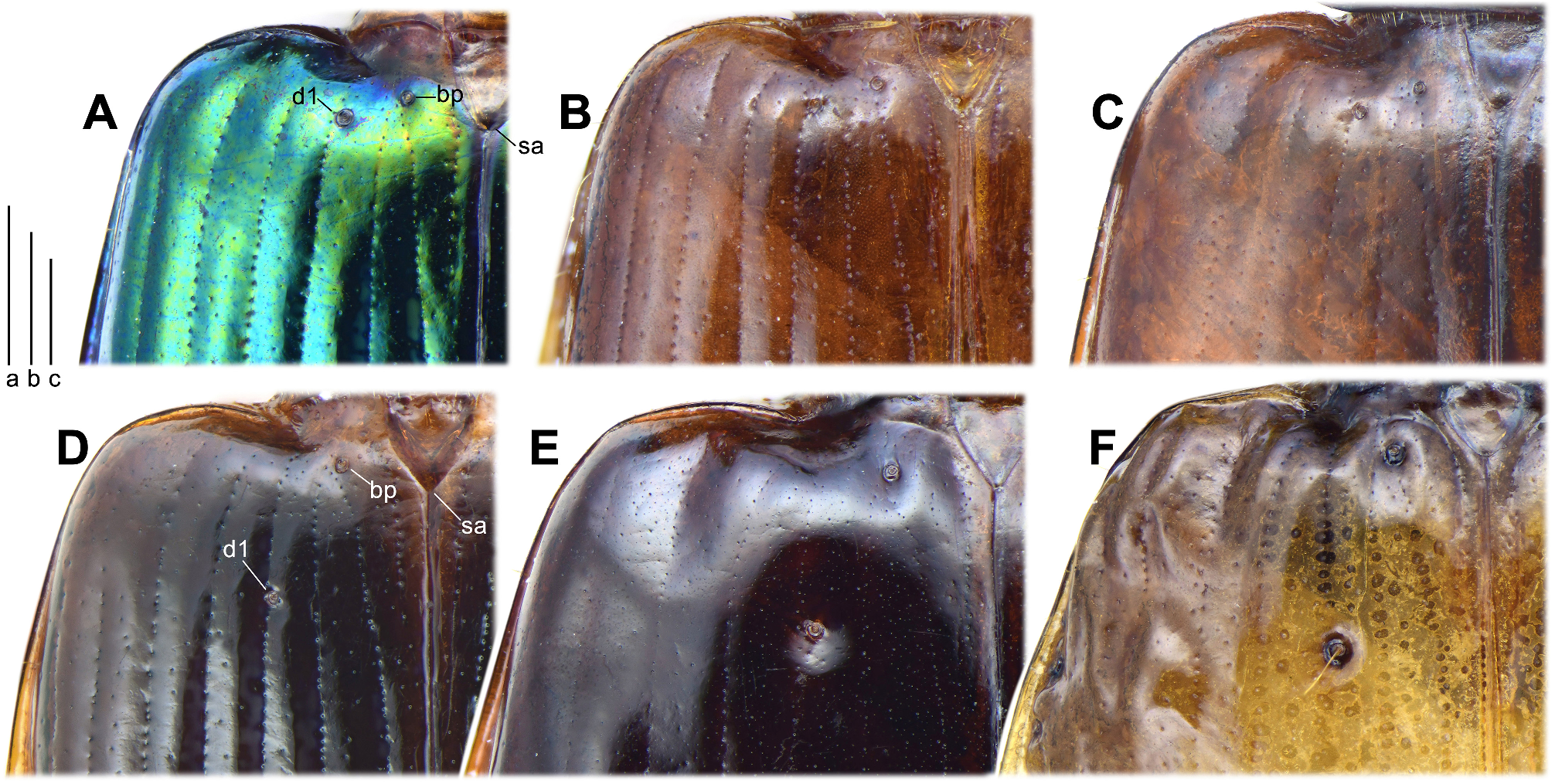
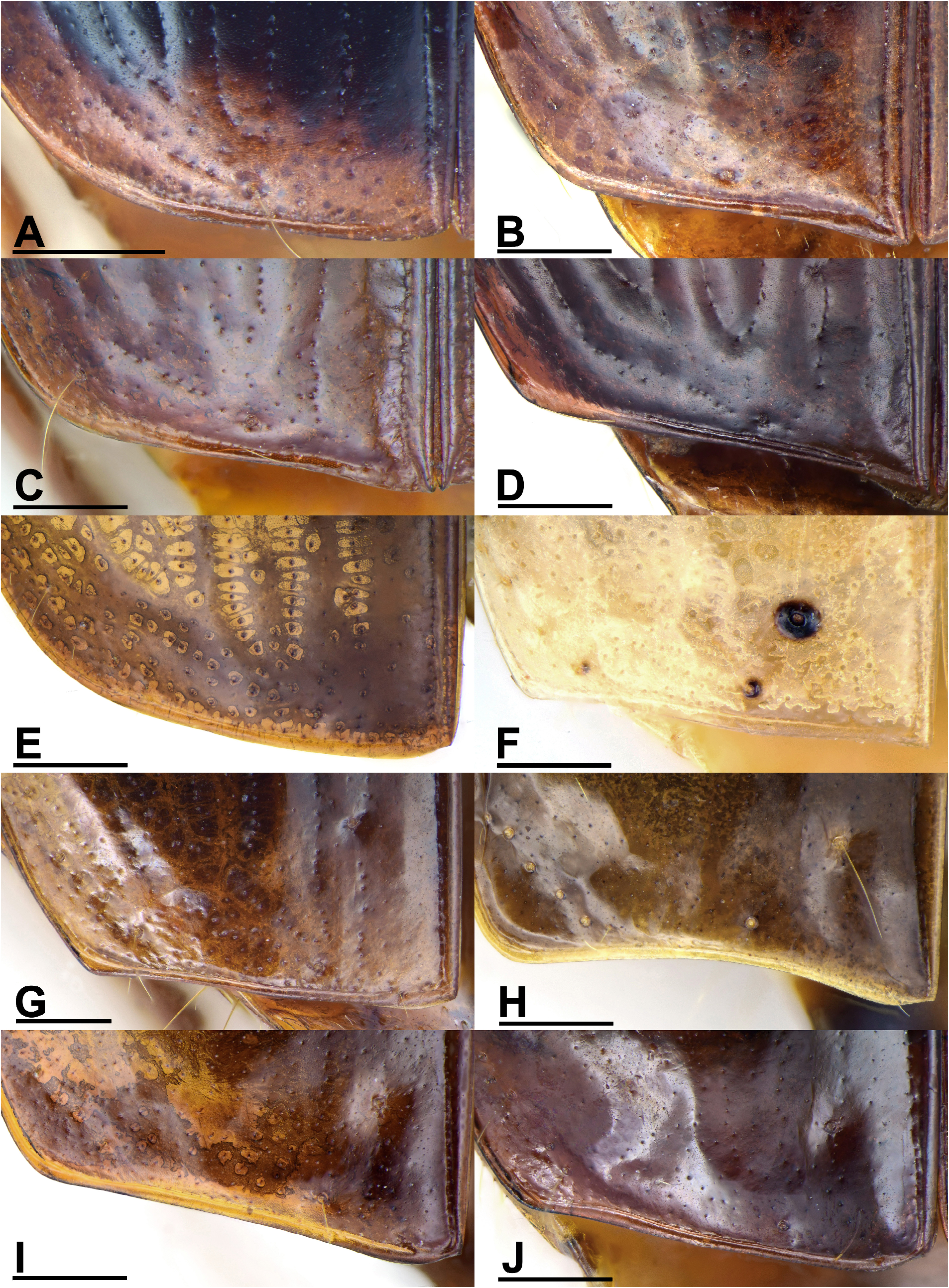
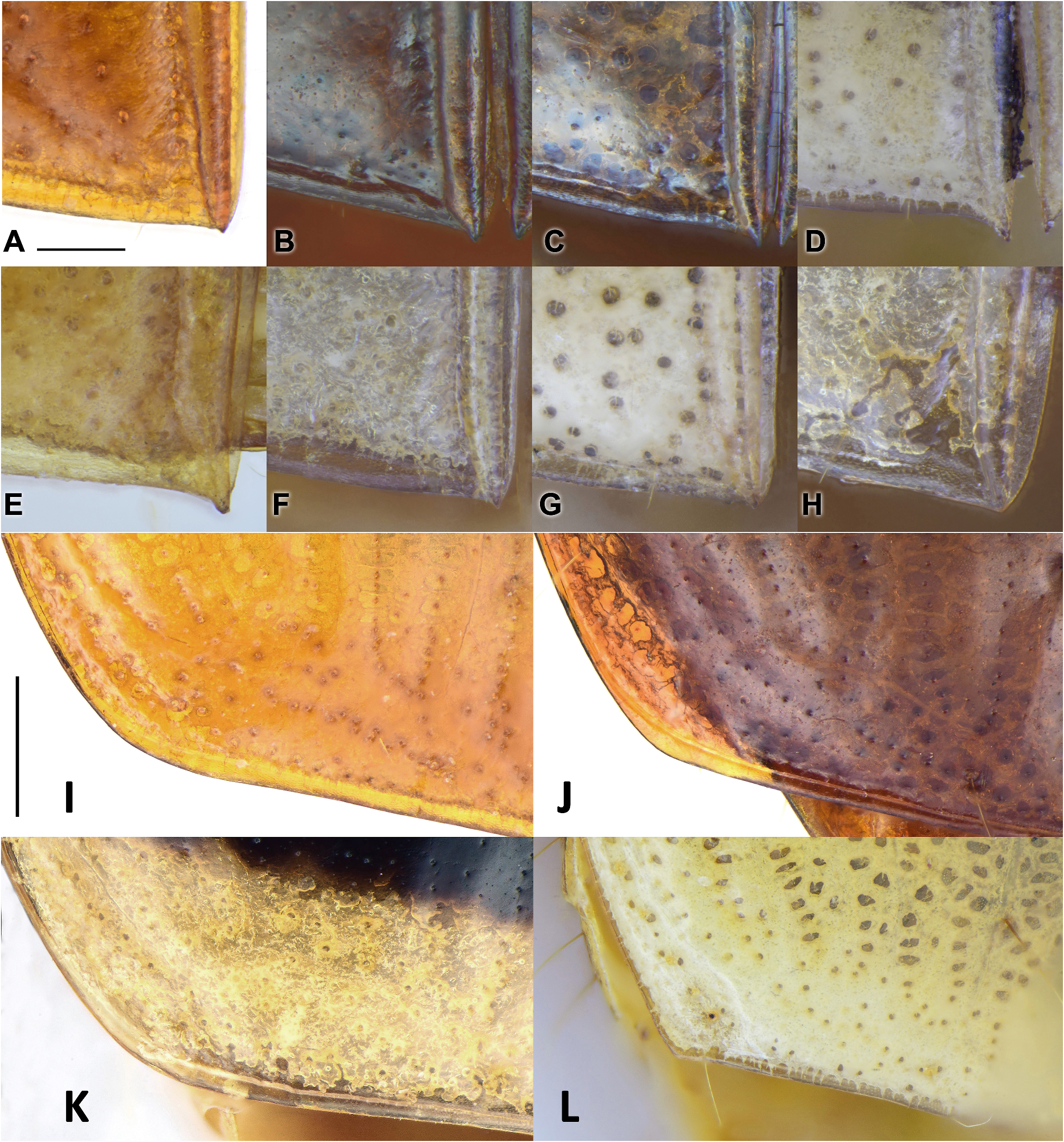
Elytra and hind wings: Elytra broad and slightly widened apically. Humeri distinct, widely rounded, without a humeral denticle. Basal ridge incomplete, extended medially only to base of the fourth stria. Elytral disc with a pair of large and shallow depressions near the middle of the interval 3 in many species, absent from several species belonging to different subgenera. In ground plan, the discal depressions are elongate or subtriangular, occupying intervals 3 to 6 and one-third to half of the elytra length. Lateral margins are usually shallowly depressed near the basal third. Intervals 7 and 8 more or less tumid near the apex and elytral surface otherwise even; but in P. heteronycha sp. n., humps are present on the base and middle parts of the lateral intervals. Scutellar striae present on interval 1, of similar depth as normal striae, usually with free apex. Basal pore (bp) present adjacent to stria 1, slightly anterior to apex of scutellum (sa). Interval 3 has three discal setigerous pores (except P. dorsigera ) with the basal two (d1, d2) adjacent to stria 3 and the apical one (d3) next to stria 2. In P. dorsigera , one additional discal pore (with two or three as individual variants) is present between d2 and d3 adjacent to stria 2. Discal pores simple, with their diameter less than half of the interval width ( Fig. 4D View FIGURE 4 ) in most species, but are strongly enlarged forming foveae greater than interval width in P. dorsigera ( Fig. 4E View FIGURE 4 ), or forming black umbilicular spots in P. heteronycha sp. n. ( Fig. 4F View FIGURE 4 ). In subgenera Crossoglossa and Parena , d1 very close to the elytra base, on the level only slightly posterior to the apex of scutellum. Thus the distance between d1 and bp is subequal or slightly less than the distance between bp and sa ( Figs 4A, 4B, 4C View FIGURE 4 ). In contrast, in the subgenus Bothynoptera , d1 is distant from the elytra base, on a level much more posterior to the apex of scutellum. Thus the distance between d1 and bp is about two times the distance between bp and sa ( Figs 4D, 4E, 4F View FIGURE 4 ). Intervals 5 and 7 are without discal pore on most species, but in a few individuals of P. dorsigera , there is one pore near the middle of interval 5. Elytra striae not or shallowly incised, finely punctate. Intervals flat or slightly convex, with very fine and sparse punctures arranged in an irregular row in most species. Stria 7 with one pore near elytral apical margin. The interval 9 less than half-width of the preceding ones. Umbilicular series composed of 18–28 pores. In the truncatipennes groups of Carabidae , the elytral apex is referred to as the apical truncation, although it is not always evidently truncate. The elytral apex is distinctly truncate in most members of the subgenus Crossoglossa , with the sutural angles usually more or less pointed ( Figs 14A–G View FIGURE 14 ), except for P. sciakyi sp. n. ( Fig. 14H View FIGURE 14 ), and the outer apical angles well rounded or weakly angulate ( Figs 14I–L View FIGURE 14 ). In the subgenera Bothynoptera and Parena , the elytral apex is subtruncate in most species, with the sutural angles not pointed and the outer apical angles well rounded ( Figs 5A, 5E View FIGURE 5 ). However, in three species groups ( P. heteronycha , P. dorsigera , and P. scutata groups), the apex is distinctly truncate, with the apical margin nearly straight or slightly concave. In the P. heteronycha and P. scutata groups, the sutural angles are more or less denticulate ( Figs 5B–D, 5F View FIGURE 5 ). In the P. heteronycha and P. dorsigera groups, the outer apical angles are prominent but apically rounded ( Figs 5F–J View FIGURE 5 ). Hind wings are fully developed, with large oblongum and wedge cells. ( Fig. 6A View FIGURE 6 )
Legs: Metacoxae with two long setae, near ante-lateral and post-lateral corners, and numerous shorter setae. Pro- and mesotrochanters each with one long seta near the apex. Metatrochanters with one long seta near the middle. All femora with four or more setae along the ventral surface. Cleaning organ of the protibiae with two cleaning setae and a well-defined cleaning spur very close to the inner margin ( Fig. 6H View FIGURE 6 ). Mesotibiae of males smooth on the ventral surface, without a notch or denticle. All tarsomeres strongly widened, with tarsomere 1 of all legs and metatarsomere 2 cylindrical, pro- and mesotarsomere 2 and tarsomere 3 of all legs subtriangular to cordate, and tarsomere 4 bilobed. Metatarsomere 1 slightly longer than metatarsomere 2. Tarsomeres sparsely setose dorsally.
In all species of the genus, the ventral surfaces of protarsomeres 1, 2, and 3 have biseriate adhesive setae along their full length. However, the adhesive setae on the ventral surface of male mesotarsomeres are always present but varied: the apical half of mesotarsomere 1 has biseriate adhesive setae on its apical portion ( Fig. 6I View FIGURE 6 ) in many species, but these setae are absent from most species of the subgenus Bothynoptera (except for P. taiwana ) and some species of the subgenus Parena ( P. latecincta , P. monticola , P. politissima , P. fulva sp. n., and P. plagiata ) ( Fig. 6J View FIGURE 6 ), or rudimentary (i.e., present as a single row near the apex) in P. circumdata . The ventral surface of mesotarsomere 2 has biseriate adhesive setae along its full length or nearly so in most species, but they are restricted to the apical half in some species of subgenera Parena ( P. politissima , P. latecincta , P. circumdata ) and Bothynoptera ( P. kurosai , P. quadrisignata , P. gonggaica sp. n., P. triguttata sp. n.). The ventral surface of mesotarsomere 3 has well-developed biseriate adhesive setae except in P. politissima . Variation in the male mesotarsal adhesive setae in different species of genus Parena is summarized in the diagram in Fig. 7 View FIGURE 7 .
Tarsal claws strongly pectinate, each with six to ten long pectinations (the medial one much smaller than others in some specimens) with the proximal one very close to the claw base ( Figs 6D–G View FIGURE 6 ). However, in the related genera Pachycallida and Metallica , there are four or five pectinations on each claw with the proximal one slightly distant from the claw base ( Figs 6B, 6C View FIGURE 6 ). In the same individual, there can be a difference of one pectination between claws in same or different legs. The tarsal claws are usually symmetric or nearly so, but conspicuously asymmetric in P. heteronycha sp. n. ( Figs 6F View FIGURE 6 , 26E–G View FIGURE 26 ). In the latter species, the inner claws of meso- and metatarsi are longer than the outer ones (the outer claws of prolegs are homologous to the inner claws of meso- and metalegs, thus the claws of prolegs are in an opposite form), with all pectinations restricted to the basal half of claw and shorter than those on the outer claw. The apical half of the inner claw is smooth and elongated. The outer claw is the same as in other species.
Venter: Pro- and mesothoracic venter glabrous or sparsely setose, metathoracic venter finely setose overall. Abdominal sternites each with numerous fine setae in addition to the primary setae. The abdominal sternite VII (terminal ventrite) with two or three primary setae (one or four in some specimens) on each side in both sexes. Apex of sternite VII of females straight in most species of the genus ( Fig. 8A View FIGURE 8 ), but weakly projected in P. mellea and P. cruralis ( Figs 8C, 8D View FIGURE 8 ) and more distinctly projected in P. sulawesiensis ( Fig. 8B View FIGURE 8 ). Apex of sternite VII of males straight ( Fig. 8E View FIGURE 8 ) in most species of subgenera Crossoglossa and Bothynoptera , but distinctly emarginate in P. mellea ( Fig. 8F View FIGURE 8 ). In most species of subgenus Parena , apex of sternite VII of males is weakly emarginate ( Fig. 8H View FIGURE 8 ), but obviously emarginate ( Figs 8I, 8J View FIGURE 8 ) in P. fasciata and P. andrewesi , or nearly straight ( Fig. 8G View FIGURE 8 ) in P. monticola , P. circumdata , and P. fulva sp. n.
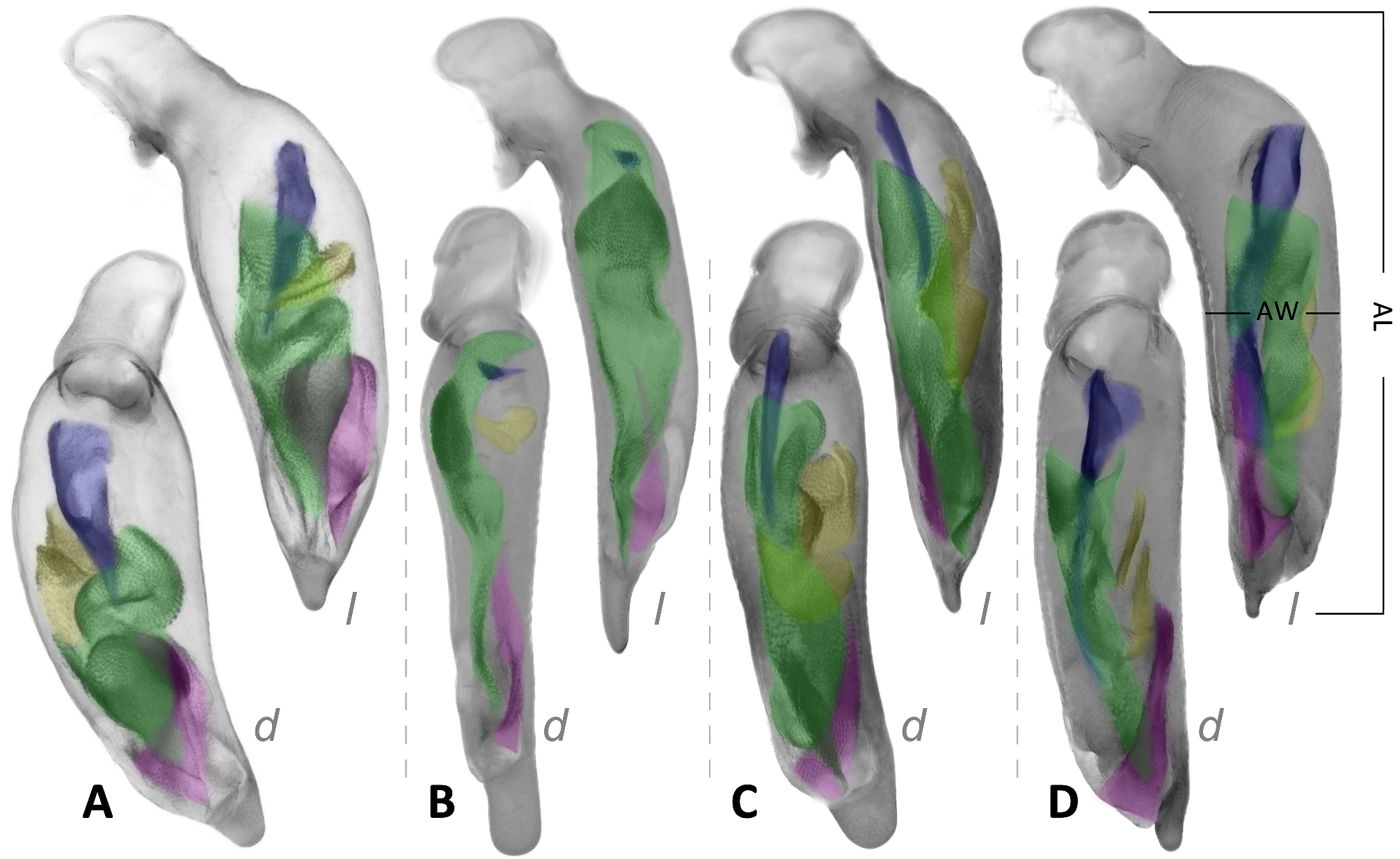
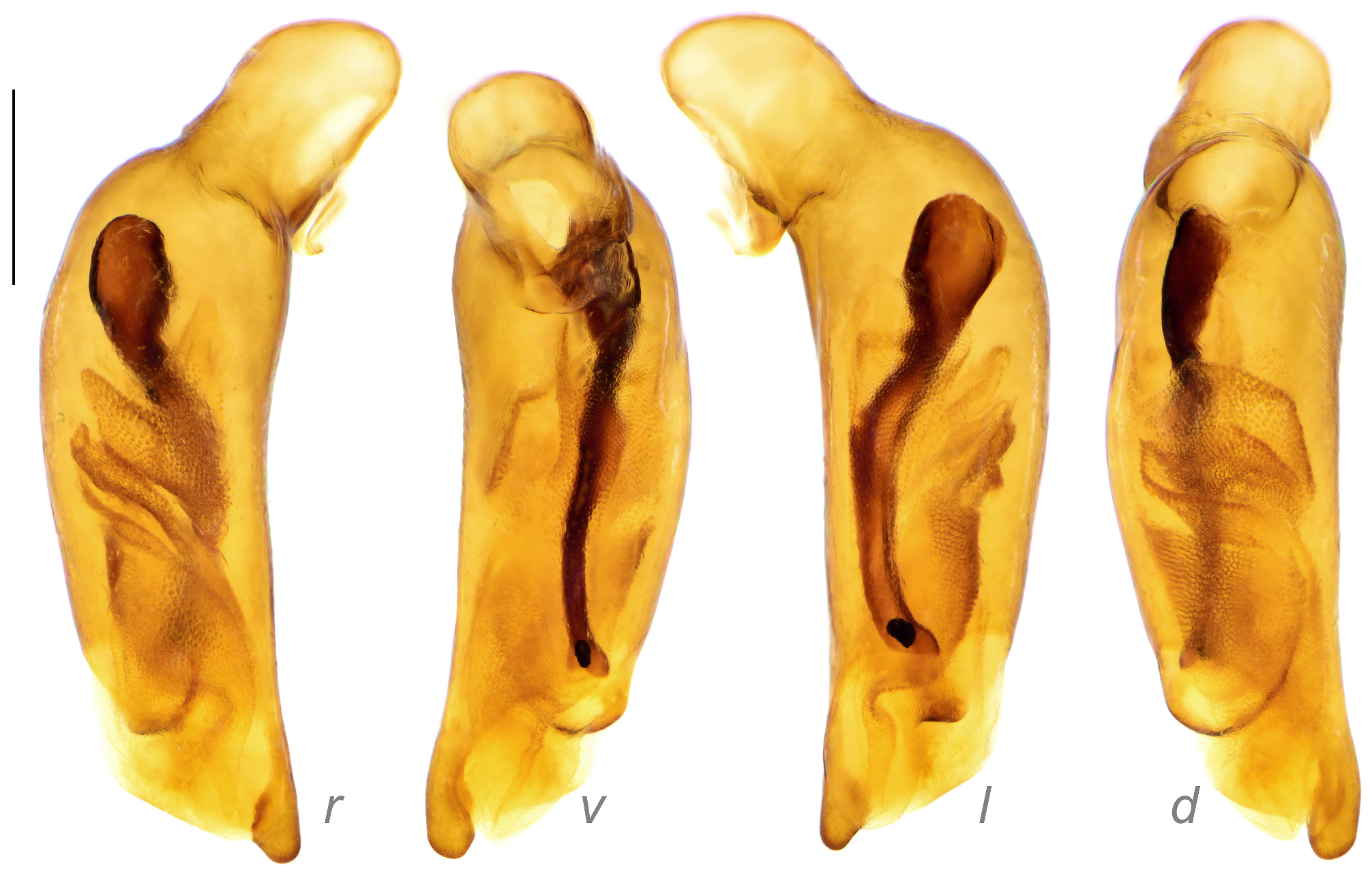
Male genitalia: Median lobe of aedeagus slightly bend near the base with shape varied. Relatively slender (AL/AW = 5.4–6.8) in subgenus Crossoglossa ; very stout (AL/AW = 3.4–4.2) in most members of subgenus Bothynoptera , but only moderately stout (AL/AW = 4.5–5.1) in the P. tesari group, or slender (AL/AW = 5.5–6.0) in the P. taiwana group; varied from very to moderately stout (AL/AW = 3.7–5.6) in subgenus Parena . Apical orifice oriented toward the left or ventral-left side of the aedeagus. Apical lamella (the apical portion between the extreme apex and the apical orifice) laminar or relatively thick, often slightly bent toward dorsum in lateral view; right margin often more or less sinuate basal to the apical lamella in dorsal view.
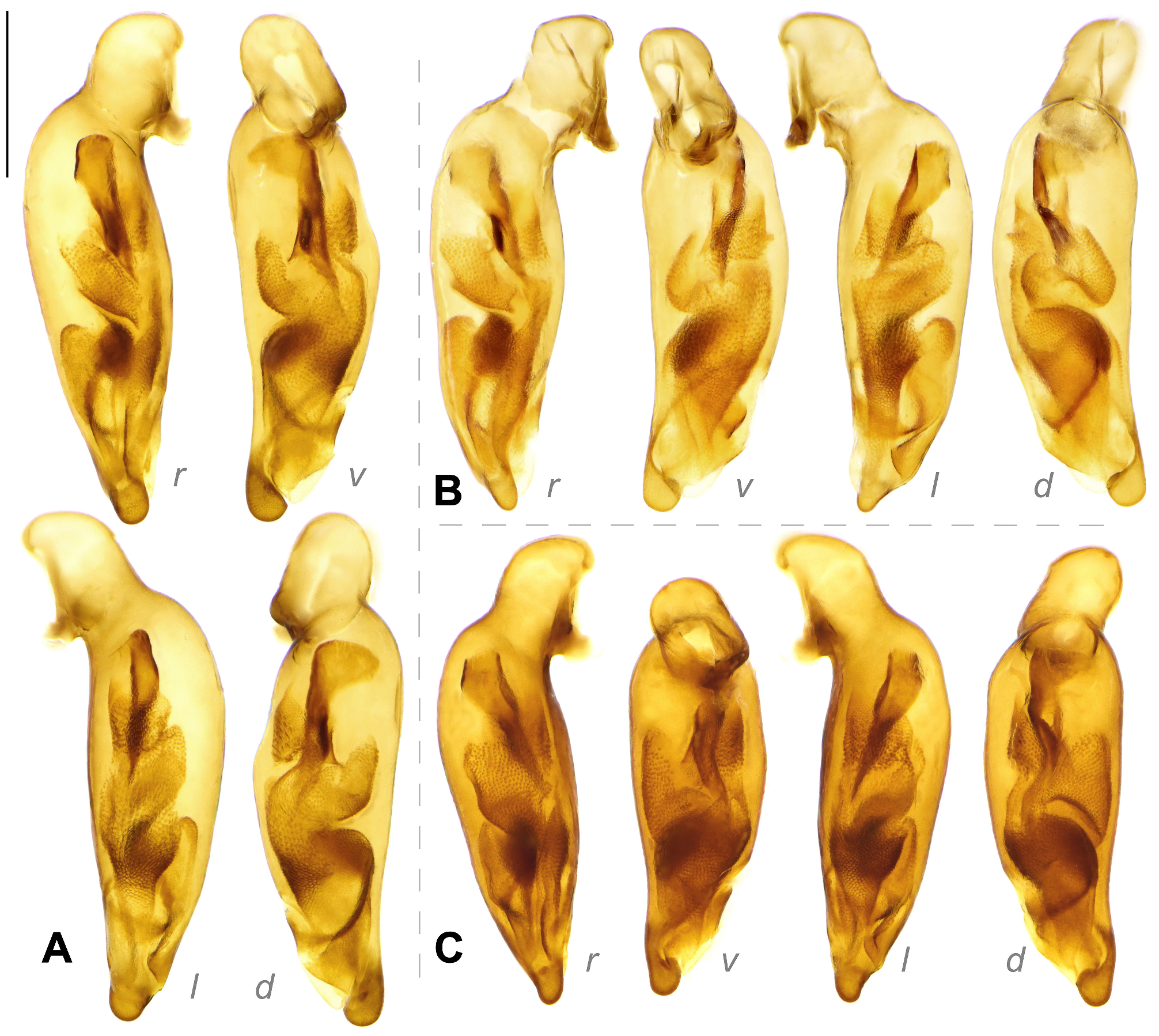
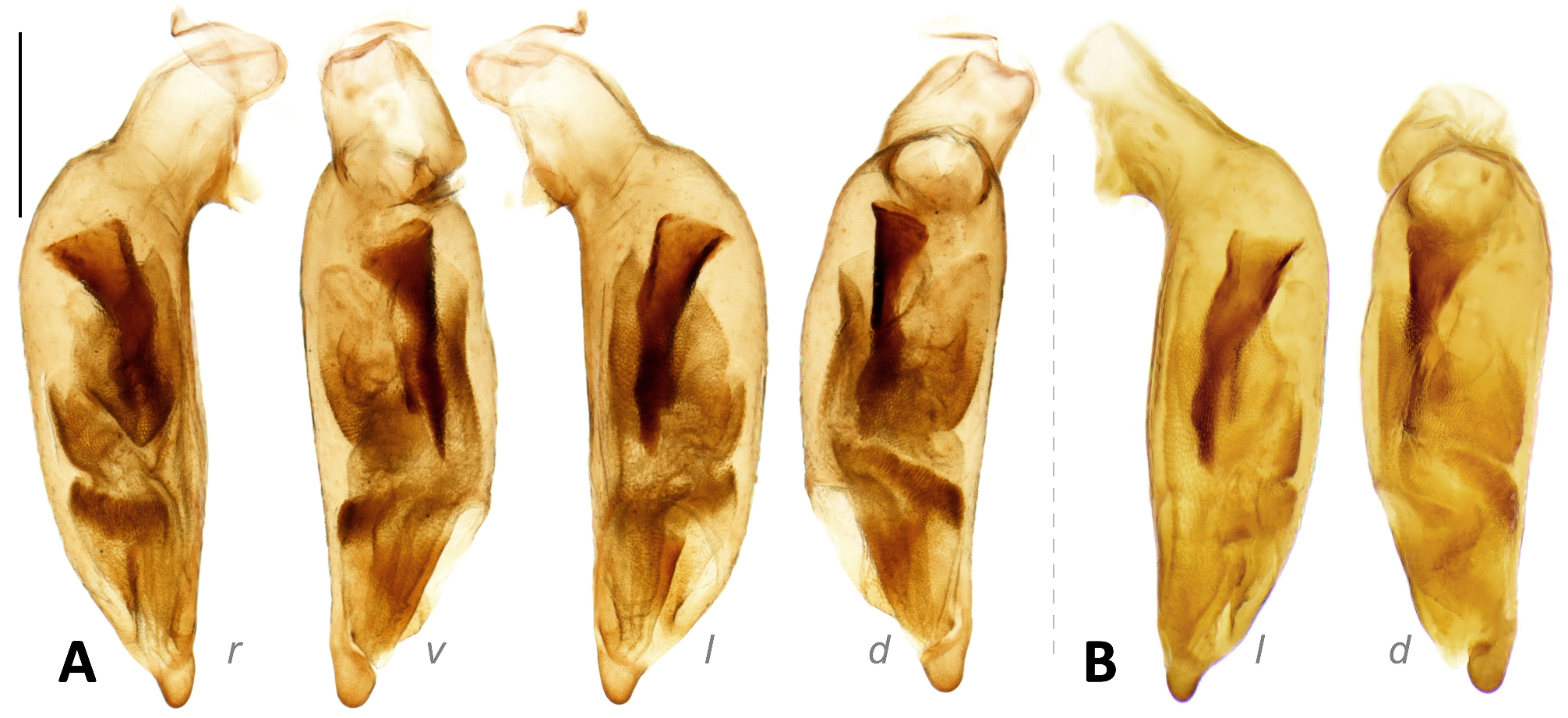
The endophallus has distinct sclerites and scaled regions, without large spines (compared to those in Metallica and Pachycallida ), with a long flagellum-like sclerite in many species, but not pointed out to the apical orifice (as in Euproctinus ). A total of four groups of structures are recognized on the endophallus. These structures exist in all species of the genus but differ in their shape. (1) Primary sclerite (blue in Fig. 9 View FIGURE 9 ) is a heavily chitinized piece, extended from the base of median lobe. It is composed of a flared basal expansion and an elongated flagellum-like apex in most species of subgenera Parena and Bothynoptera ( Figs 9A, 9D View FIGURE 9 ). The flared expansion is reduced in the P. bicolor group, thus the primary sclerite is filiform in these species ( Fig. 9C View FIGURE 9 ). The flagellum is thick and extended to the apical orifice, as in P. kurosai ( Fig. 30 View FIGURE 30 ), or thinner and gradually tapered and terminated before apical orifice such as P. tripunctata ( Fig. 9A View FIGURE 9 ). The primary sclerite is rudimentary in the subgenus Crossoglossa and is present as a very small triangular piece near the base of median lobe ( Fig. 9B View FIGURE 9 ). (2) Apical sclerite (magenta in Fig. 9 View FIGURE 9 ) is a V-shaped (in subgenera Parena and Bothynoptera , Figs 9A, 9C, 9D View FIGURE 9 ) or ribbon-form (in subgenus Crossoglossa , Fig. 9B View FIGURE 9 ) piece near the apical orifice. It is less chitinized than the primary sclerite. Sometimes the major portion of the apical sclerite is weakly defined (such as in P. kurosai ), but its left edge is always well chitinized. The basal core refers to the darkest area near the base of the apical sclerite which is usually heavily scaled. It is usually ovate or strongly elongated, but extended as a transverse scaled belt as in P. fulva ( Fig. 75 View FIGURE 75 ), or indistinct as in P. kurosai ( Fig. 30 View FIGURE 30 ). (3) Squamate sheath (green in Fig. 9 View FIGURE 9 ) is the large scaled membranous portion extended from the base of median lobe to the apical orifice. It usually surrounds the apical part of the primary sclerite. There is a gap in the middle of the squamate sheath, dividing it into a basal sheath and an apical sheath. The apical sheath is usually much larger and more heavily scaled than the basal sheath. (4) Squamate sac (yellow in Fig. 9 View FIGURE 9 ) refers to the small scaled sac present on the basal half of median lobe, well separated from the squamate sheath. It is usually on the dorsal side to the squamate sheath, sometimes turns to the right or left side. The squamate sac is simple (such as in P. malaisei , Fig. 44 View FIGURE 44 ) or divided into two sacs (such as in P. tripunctata , Fig. 9A View FIGURE 9 ): The proximal sac is usually like a scaled membranous sac ( Fig. 9D View FIGURE 9 ) but sometimes has a dentate chitinized apex as in P. bicolor ( Fig. 9C View FIGURE 9 ). The distal sac is usually the same size as the proximal one, but less chitinized.
Left paramere large and rounded, with concave outer surface and rounded or slightly truncate apex. The right paramere is smaller than the left and trifurcate with the apex varied ( Fig. 10 View FIGURE 10 ). The parameres have very little taxonomical value in the genus Parena because they are similar among different species and the apices are somewhat varied infraspecifically.
Female genitalia: Gonocoxite I of ovipositor without setae or spines, with its dorsal surface strongly concave in subgenus Crossoglossa , shallowly concave in subgenera Parena and Bothynoptera . Gonocoxite II subquadrate or dichotomous, with four to seven ensiform setae on the apex, without nematiform seta or membranous apical extension.
In subgenus Crossoglossa , gonocoxite II dichotomous and strongly bent outwards forming a U-shaped structure ( Figs 11Y View FIGURE 11 –γ). The inner branch with two ensiform setae on the apex. The outer branch slightly longer than the inner one in the P. laesipennis group ( Fig. 11Z View FIGURE 11 ), and similar length in the other two groups. It with two or three ensiform setae on the apex, of similar length to those on the inner branch in the P. testacea group ( Figs 11 View FIGURE 11 α–γ) and much shorter in the other two groups ( Figs 11Y, 11Z View FIGURE 11 ).
In subgenera Parena and Bothynoptera , gonocoxite II quadrate or nearly so, with the apex concave, nearly straight, or slightly convex ( Figs 11A–X View FIGURE 11 ). The apex with an evident tooth near the inner angle (e.g., Fig. 11O View FIGURE 11 ) in many species, but the tooth very faint in most species of Bothynoptera and in P. fulva sp. n. (e.g., Fig. 11U View FIGURE 11 ). The ventral surface of gonocoxite II with a large depression (e.g., Fig. 11S View FIGURE 11 ) in most species, but the depression very shallow in subgenus Bothynoptera and in the P. scutata group of subgenus Parena (e.g., Fig. 11V View FIGURE 11 ). The apex with four to seven ensiform setae. In most species of subgenus Bothynoptera , these setae are subequally arranged with two or three of them grouped on the outer angle (e.g., Fig. 11B View FIGURE 11 ); but in P. kurosai and P. tesari (probably also P. obscura ), there are three or four ensiform setae grouped on the inner and outer angles, respectively ( Figs 11C, 11D View FIGURE 11 ). In most species of subgenus Parena , there are two or three ensiform setae on the outer angle, two on the inner angle, and another one next to the apical tooth (e.g., Fig. 11N View FIGURE 11 ); but in P. fulva sp. n., these setae are nearly equally spaced except the inner two slightly close to each other ( Fig. 11U View FIGURE 11 ). In the P. bicolor group, P. scutata group, and the other two species of the P. stigmatica group, all of these setae are grouped to the inner and outer angles (e.g., Fig. 11K View FIGURE 11 ), but one seta of each group may be slightly distant from others (e.g., Fig. 11W View FIGURE 11 ) in some species.
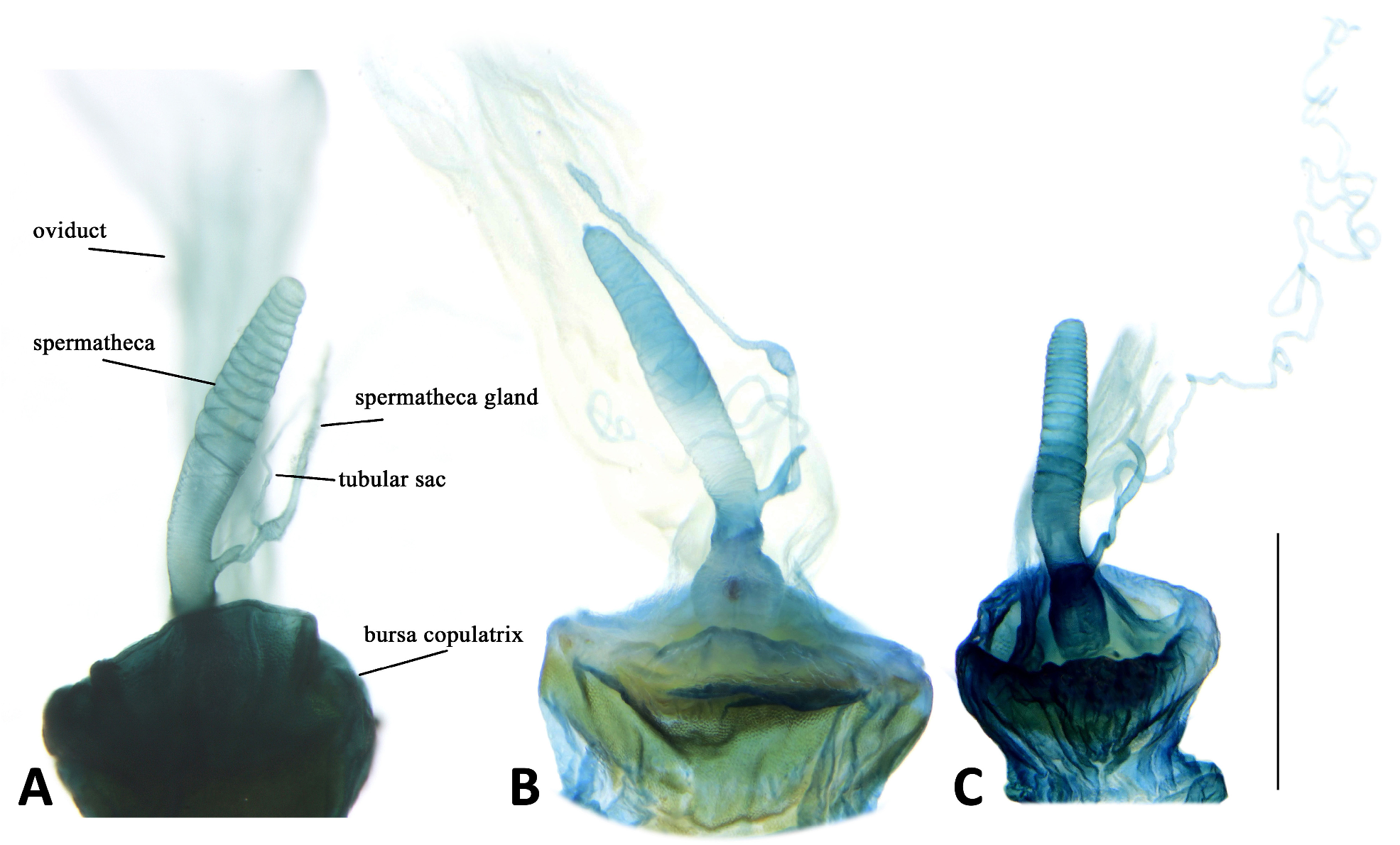
Female internal reproductive tracts are similar among different subgenera of Parena ( Fig. 12 View FIGURE 12 ). Spermatheca digitiform without a differentiated pedicel, strongly whorled on the surface of the apical half, inserted on the dorsal surface of bursa copulatrix close to the junction of common oviduct. Spermathecal gland similar in length to spermatheca, more or less branched near base, inserted to the lateral side of spermathecal base. The tubular sac slender and much longer than spermathecal gland, inserted near the base of spermathecal gland.
Monophyly and relationships.
The subtribe Metallicina was erected by Basilewsky (1984) for Metallica , Pachycallida and Parena . Soon after that, Euproctinus was included in Metallicina as well ( Shpeley, 1986).
As the result of a preliminary phylogenetic analysis ( Shpeley, 1986), the monophyly of the subtribe Metallicina can be suggested by the following synapomorphic character states: antennomeres 5–11 each with ventral sensory pits; apex of glossal sclerite with four or more apical setae; head with suborbital setae; antennomere 1 with a carina. Within the subtribe Metallicina , the genus Parena is presumed to be the sister group of Metallica + Pachycallida , supported only by one synapomorphic character state: the retinacular ridge of right mandible reduced. Nevertheless, this relationship seems reliable as it fits the zoogeographical pattern as well.
The monophyly of genus Parena could be supported by the following apomorphic character states: (1) male genitalia with endophallic sclerite; (2) gonocoxite II of ovipositor with four or more ensiform setae, without membranous apex or trichoid setae; (3) female reproductive tracts with extremely long tubular sac. Three subgenera are recognized under the genus Parena . The monophyly of two subgenera ( Crossoglossa and Bothynoptera ) seems to be well supported, whereas the third ( Parena s. str.) could be paraphyletic. Detailed discussions on the infrageneric relationships are provided under each subgenus.
Life history and larvae features.
Adults of Parena can be found on leaves or trunks of various woody plants. They are frequently collected by beating dense vine plants or dead leaves hanging on small trees. They might be hidden inside the dead leaf rolls in the daytime and active at the night preying mainly on lepidopteral larvae. Many species are attracted by lights, whereas a few very rare species of the subgenus Bothynoptera were only collected from the canopy of trees with height between 5 and 8 m.
Based on the literature by Andrewes, Habu, Yokoyama, Nawa, Minamikawa, etc., Habu (1967) reviewed the life history of some common Japanese species: the adults of P. nigrolineata nipponensis (= P. nigrolineata ) are reported to prey on various lepidopteral larvae including the silk worm ( Bombyx mori L.); the larvae of P. perforata (= P. dorsigera ) are predatory on Spilosoma imparilis Butler (Arctiidae) ; P. cavipennis is predaceous on Phrixolepia sericea Butler (Heterogeneidea) ; the adults of P. tripunctata were found in the leaf-rolls of Paroplapoderus pardalis Vollenhoven ( Attelabidae , Coleoptera ), but it remains to be proven whether or not they feed on the weevils. More recently, Habu (1981) reported that P. cavipennis is predaceous on Scopelodes contracta Walker (Heterogeneidae) . Mochizuki (1990) reported that P. laesipennis feeds on larvae of Pidorus glaucopis (Drury) (Zygaenidae) .
In India, P. nigrolineata was reported as a predator of Opisina arenosella Walker (Oecophoridae) , the coconut caterpillar ( Mohamed et al., 1982; Pushpalatha & Verresh, 1995), Nephopteryx eugraphella Ragonot (Pyralidae) , the chiku moth ( Sran & Sandhu, 1979), and Atteva fabriciella Swederus (Yponomeutidae) , the ailanthus webworm ( Misra, 1994).
In China ( Shandong) and Korea, P. cavipennis was reported as an important predator of Hyphantria cunea (Drury) , the fall webworm ( Ko & Ko, 1982; Wang et al., 1999). Both adults and larvae can attack larvae of the webworm. Zhao et al. (2011) reported P. cavipennis as a predatory natural enemy of Antheraea pernyi (Guérin-Méneville), the tussah silkworm, in Liaoning province. Larvae of the beetle bite the ventral side of the silkworm, sucking its body fluids and causing death in days. P. cavipennis was also reported as predaceous on larvae of various lepidopteral pests, including twenty-eight species belonging to ten families, and Arge captiva (Smith) ( Argidae , Hymenoptera ) ( Zhou, 1988; Zhao et al., 2011). P. latecincta was reported as predaceous on Termioptycha bilineata (Wileman) (Pyralidae) , an important pest on the staghorn sumac in Beijing. Both larvae and adults of the beetle can attack pyralid moth larvae inside their leaf rolls ( Zhao et al., 2014).
In China ( Liaoning), P. cavipennis hibernates in the adult stage in dry habitat under trees, such as under dirt clumps, rocks, or dead leaves. Adults are active between May and October. The egg stage starts from middle July, lasting about five days. The average stadia of larvae, prepupa and pupa are 9–12d, 2–3d, 8–10d, respectively ( Zhao et al., 2011).
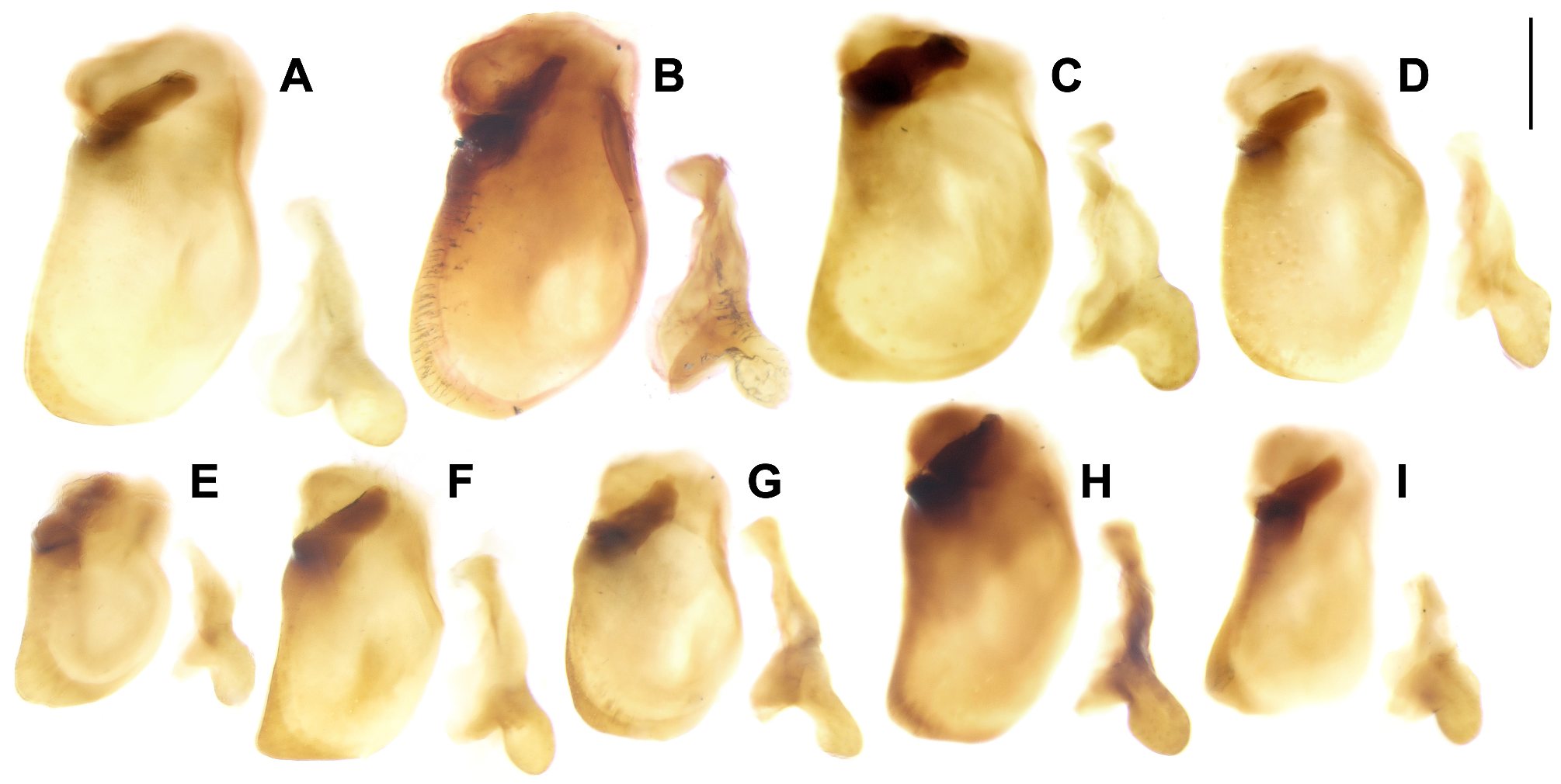
Larvae of some East Asian species have been illustrated and described by different authors: Habu et al. (1963) for P. nigrolineata nipponensis (= P. nigrolineata ); Habu & Sadanaga (1967) for P. perforata (= P. dorsigera ); Habu (1981) for P. cavipennis ; Hondo (2012) for P. tripunctata ; and Zhao et al. (2014) for P. latecincta .
Diversity and biogeography
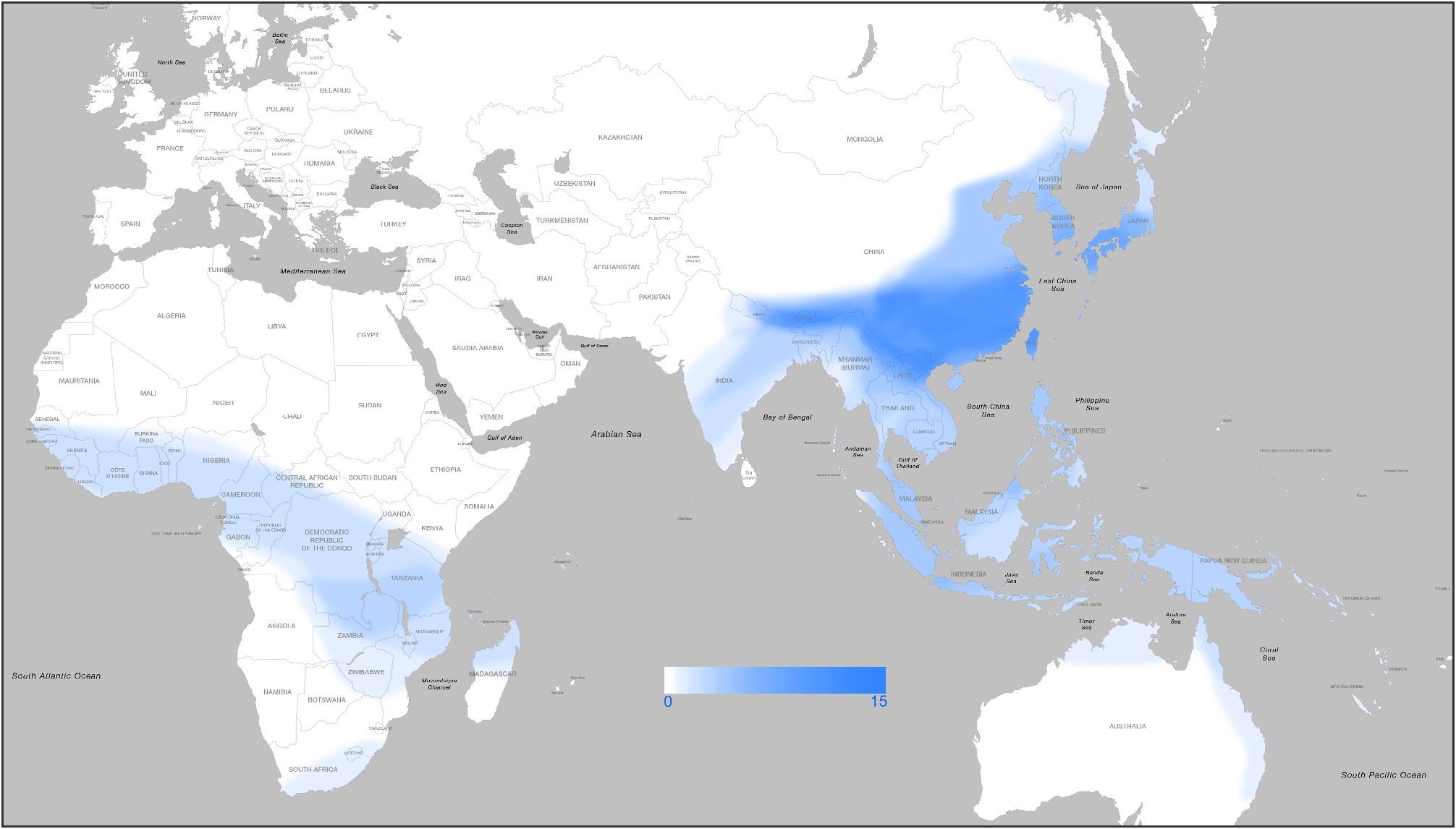
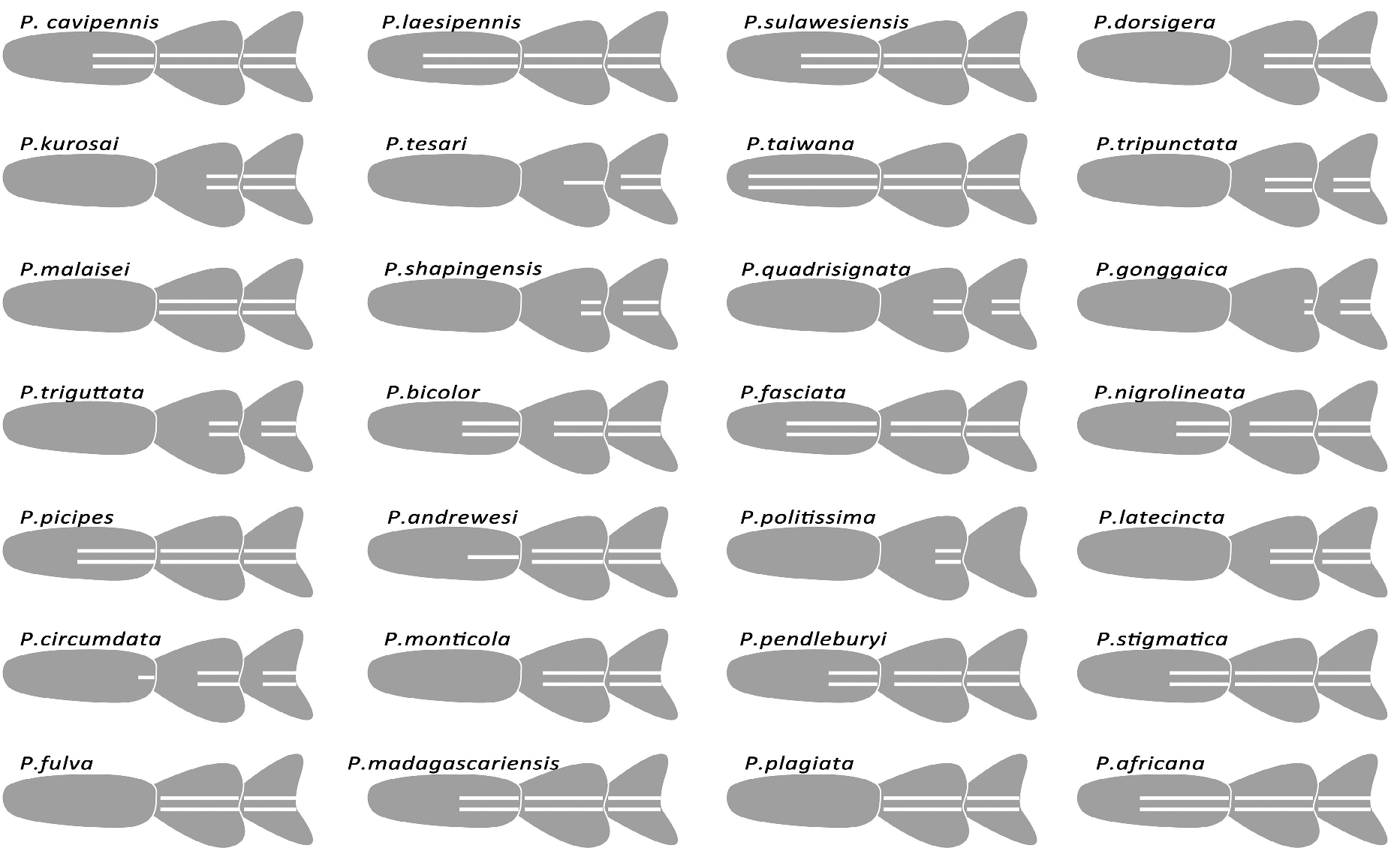
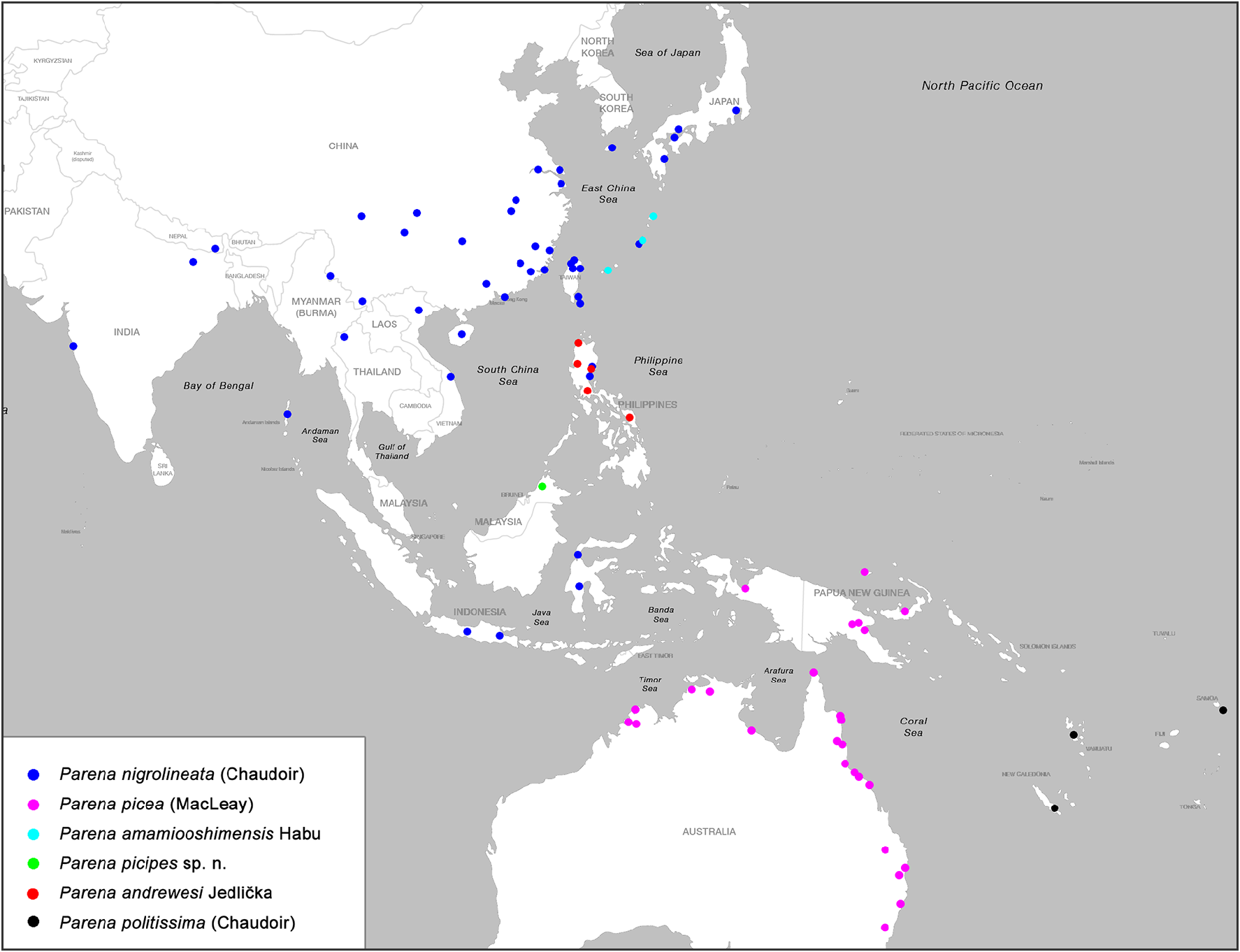
The genus Parena is composed of 46 species classified into three subgenera. This genus has an extensive distributional range in the Old World Tropics, from West Africa eastward through Madagascar, India, and Indochina to the Malay Archipelago, and easternmost to New Caledonia and Samoa; southward to the eastern coastal region of Australia; and northward through East Asia to Primorye, Russia. The specific diversity of genus Parena from Asia is far richer than that from Africa or Australia. The highest species richness occurs in south China and on the south slope of the Himalayan Range ( Map 1 View MAP 1 ). For example, in some regions (e.g., southern Yunnan and Taiwan) of southern China, as many as thirteen species occur in an area of no more than ten thousand square kilometers.
Among the three subgenera of Parena , the nominotypical subgenus has the widest distributional range, reaching from Africa and Australia. The other two subgenera are confined to the Oriental Realm, the eastern part of Palaearctic Realm, and Papua New Guinea. The subgenus Crossoglossa is most diverse in the Malay Archipelago, and the subgenus Bothynoptera ranges farther north with its highest diversity in south China.
Members of some species groups have strictly allopatric distributions, showing examples of geographical replacement. For example, in the P. tesari species group ( Map 7 View MAP 7 ), P. tesari (Jedlička) is widely distributed in south China, while its adelphotaxon, P. obscura Mateu , is only from Bhutan and Nepal. In the P. nigrolineata species group ( Map 10 View MAP 10 ), P. nigrolineata (Chaudoir) is widely distributed in the area west to Weber's Line, while its adelphotaxon P. picea (Macleay) is from the area east of Weber's Line. In the species groups of P. laesipennis , P. testacea , P. scutata , and P. plagiata , all species are strictly allopatric with some very widely distributed and others restricted to small areas.
Although some species are very common and widely distributed (e.g., P. nigrolineata from Asia, and P. africana from African Continent), several species in the genus are relatively rare in collections. Some of these rare species are very narrowly distributed (e.g., P. picipes sp. n. and P. pendleburyi Andrewes are endemic to north Borneo, and P. heteronycha sp. n. is endemic to north Laos). A few species are only known from the holotype material (e.g., P. gonggaica sp. n. from Sichuan, and P. dorae Basilewsky from Angola), however some others are relatively widely distributed but still known from only very few specimens (e.g., P. monticola Shibata from China, and P. fulva sp. n. from Southeast Africa). It seems to be very difficult to collect these rare species on low vegetation, because individuals of some species (e.g., P. emarginata sp. n. from China) of the subgenus Bothynoptera were only collected from the canopy of medium-sized trees, by using a long-stick sweeping net.
Species excluded from genus Parena
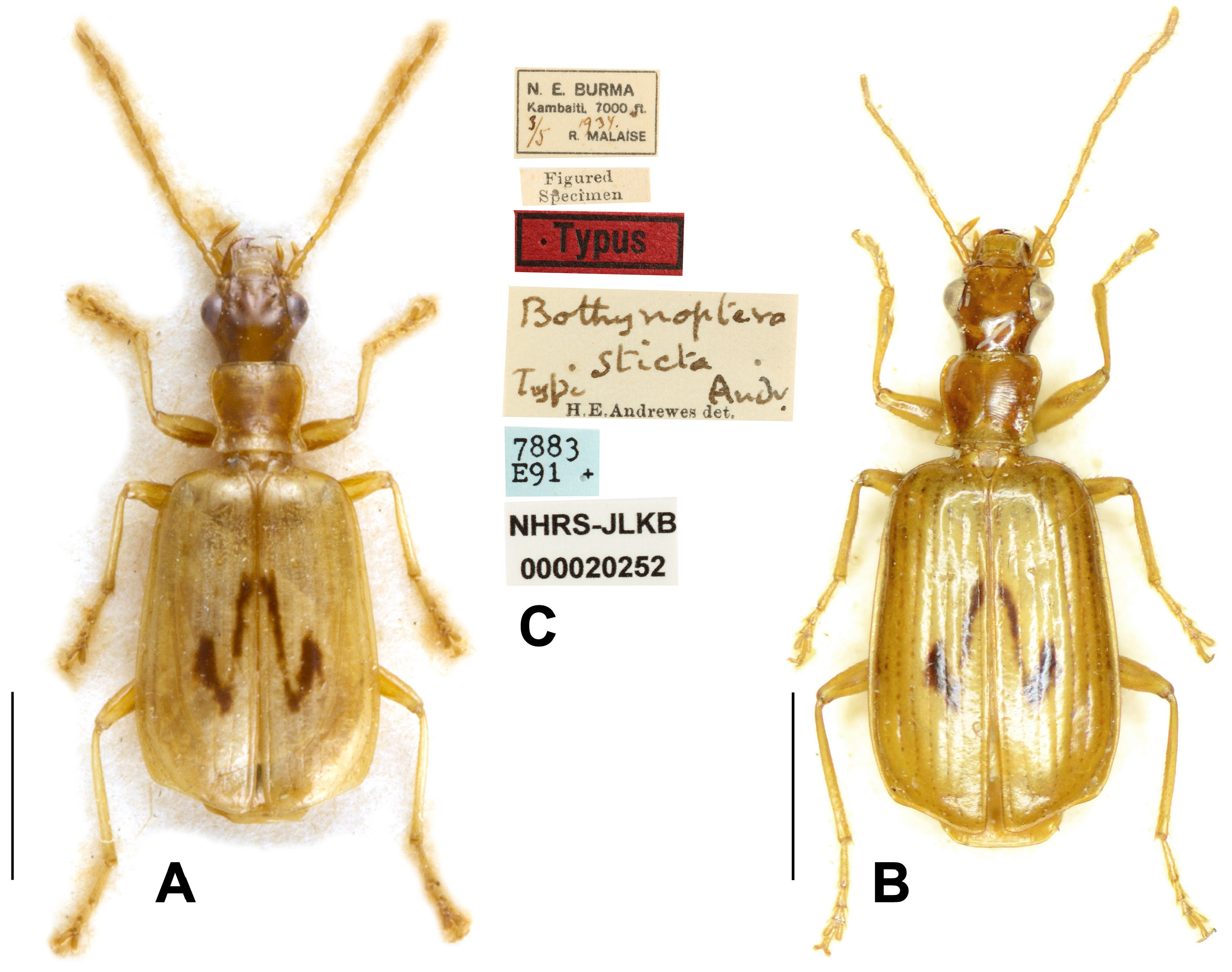
Andrewes (1947) described Bothynoptera sticta based on four specimens collected from Kambaiti in Burma. This species was mentioned by Jedlička (1963) but its taxonomic status has not been discussed after that. In recent catalogues ( Lorenz, 2005; L̂bl & L̂bl, 2017), it was placed in the genus Parena .
A few years ago, the senior author examined one paratype ( Fig. 13B View FIGURE 13 ) of this species in NHML, and later, the holotype ( Fig. 13A View FIGURE 13 ) and another paratype deposited in NHRS were examined through photos thanks to the help of Dr. J. Bergsten. Based on these, we found that this species is different from all members of the genus Parena in the following aspects: (1) mandibles not widened, outer margins nearly straight; (2) interval 3 of elytra with only two setigerous pores; (3) terminal palpomeres acuminate to apex; (4) eyes not hemispheric, much less convex than other species of Parena ; and (5) metatarsomere 1 longer than the combination of following two tarsomeres. Although we didn’t study the ventral or genital characters of this species, all the above character states support well that this species actually belongs to the genus Peliocypas Schmidt-Ĝbel. We propose the new combination herein: Peliocypas stictus ( Andrewes, 1947) comb. nov.
Materials examined. Holotype (NHRS): examined by photo, "N.E. BURMA / Kambaiti, 7000 ft. / 3/5 1934 / R. MALAISE", " Bothynoptera / sticta / Type Andr. / H.E. Andrewes det.". "Typus" [red label], "Figured / Specimen", "7883 / E91+" [blue label], "NHRS-JLKB / 000020252" < Figs 13A, 13C View FIGURE 13 >. Paratypes: 1 ex (NHRS), examined by photo, "N.E. BURMA / Kambaiti, 7000 ft. / 4-8/6 1934 / R. MALAISE", " Bothynoptera / sticta / Cotype Andr. / H.E. Andrewes det.". " Paratypus " [red label], "7884 / E91+" [blue label], "NHRS-JLKB / 000020253". 1 ex (NHML), "N.E. BURMA / Kambaiti, 7000 ft. / 20/6 1934 / R. MALAISE", " Bothynoptera / sticta / Cotype Andr. / H.E. Andrewes det.". "Co- / type" [round label with green circle label] < Fig. 13B View FIGURE 13 >.
Checklist of genus Parena Motschulsky, 1860
Subgenus Crossoglossa Chaudoir, 1872
1. Parena cavipennis species group
[1] Parena cavipennis ( Bates, 1873)
rufotestacea Jedlička, 1934 syn. nov.
2. Parena laesipennis species group
[2] Parena laesipennis ( Bates, 1873)
[3] Parena levata Andrewes, 1931
[4] Parena obenbergeri Jedlička, 1952
3. Parena testacea species group
[5] Parena testacea ( Chaudoir, 1872)
[6] Parena sulawesiensis Kirschenhofer, 2006
[7] Parena mellea ( Chaudoir, 1872)
[8] Parena cruralis Andrewes, 1935
[9] Parena sciakyi sp. nov.
Subgenus Bothynoptera Schaum, 1863
4. Parena heteronycha species group
[10] Parena heteronycha sp. nov.
5. Parena dorsigera species group
[11] Parena dorsigera ( Schaum, 1863)
perforata ( Bates, 1873) syn. nov.
nepalensis Kirschenhofer, 1994 syn. nov.
kunmingensis Kirschenhofer, 1996 syn. nov.
[12] Parena kurosai Habu, 1967
wrasei Kirschenhofer, 2006 syn. nov.
6. Parena tesari species group
[13] Parena tesari ( Jedlička, 1951)
nantouensis Kirschenhofer, 1996 syn. nov.
kataevi Kirschenhofer, 2006 syn. nov.
[14] Parena obscura Mateu, 1977
7. Parena taiwana species group
formosana Ohkura, 1978 [homonym]
8. Parena tripunctata species group
[16] Parena tripunctata ( Bates, 1873)
[17] Parena shapingensis Xie & Yu, 1993
yunnana Kirschenhofer, 1994 syn. nov.
[18] Parena monostigma ( Bates, 1873)
japonica Jedlička, 1946
koreana Kirschenhofer, 1994 syn. nov.
[19] Parena malaisei ( Andrewes, 1947)
albomaculata Habu, 1979 syn. nov.
[20] Parena quadrisignata Mateu, 1977
phongsalyensis Kirschenhofer, 2011 syn. nov.
[21] Parena emarginata sp. nov.
[22] Parena gonggaica sp. nov.
[23] Parena triguttata sp. nov.
Subgenus Parena Motschulsky, 1860
Oriental-Australian species 9. Parena bicolor species group [24] Parena bicolor Motschulsky, 1860 [25] Parena rubripicta Andrewes, 1928 [26] Parena fasciata ( Chaudoir, 1872) plagiata (Macleay, 1876) sloanei Csiki, 1932 sellata ( Heller, 1921) syn. nov.
hastata ( Heller, 1921) syn. nov.
sellatoides ( Jedlička, 1940) syn. nov.
unicolor Louwerens, 1949 syn. nov.
sumatrana Kirschenhofer, 2011 syn. nov.
10. Parena nigrolineata species group
[27] Parena nigrolineata ( Chaudoir, 1852)
nipponensis Habu, 1964 syn. nov.
schillhammeri Kirschenhofer, 2006 syn. nov.
[28] Parena picea ( Macleay, 1871)
[29] Parena amamiooshimensis Habu, 1964
[30] Parena picipes sp. nov.
[31] Parena andrewesi Jedlička, 1934
[32] Parena politissima ( Chaudoir, 1883)
11. Parena latecincta species group
[33] Parena latecincta ( Bates, 1873)
viridilineata ( Jedlička, 1939)
[34] Parena circumdata Shibata, 1987
[35] Parena monticola Shibata, 1987
[36] Parena pendleburyi Andrewes, 1931
African species
12. Parena stigmatica species group
[37] Parena stigmatica ( Fairmaire, 1899)
[38] Parena dorae Basilewsky, 1955
[39] Parena fulva sp. nov.
13. Parena scutata species group
[40] Parena madagascariensis ( Alluaud, 1917)
alluaudi Jeannel, 1949 syn. nov.
[41] Parena valeriae Facchini, 2011
[42] Parena scutata ( Alluaud, 1917) stat. res.
[43] Parena ruficornis sp. nov.
14. Parena plagiata species group
[44] Parena ferruginea ( Chaudoir, 1878)
[45] Parena plagiata Motschulsky, 1864
formidulosa ( Peringuey, 1898)
[46] Parena africana ( Alluaud, 1917)
Key to subgenera of genus Parena View in CoL
1. Pronotum strongly transverse (PW/PL = 1.50–1.82, generally greater than 1.60), distinctly wider than head (PW/HW = 1.05– 1.26, generally greater than 1.10); mentum without seta, lateral lobes extremely large, inner margin nearly straight, epilobes very narrow ( Fig. 3A View FIGURE 3 ); gonocoxite II of ovipositor dichotomous, each branch with two or three ensiform setae apically ( Figs 11Y View FIGURE 11 –γ); median lobe of aedeagus slender, endophallus with very small primary sclerite near base ( Fig. 9B View FIGURE 9 )......................................................................................... subgen. Crossoglossa Chaudoir View in CoL
- Pronotum less transverse (PW/PL = 1.19–1.55, generally less than 1.50), narrower or barely wider than head (PW/HW = 0.85–1.13, generally less than 1.10); mentum with a pair of setae (in some species very short), lateral lobes relatively short, inner margin more or less oblique, epilobes rather wide ( Figs 3B–D View FIGURE 3 ); gonocoxite II of ovipositor nearly quadrate, apex with five to seven ensiform setae ( Figs 11A–X View FIGURE 11 ); median lobe of aedeagus stouter, endophallus with large primary sclerite ( Figs 9A, 9C, 9D View FIGURE 9 )................................................................................................ 2
2. First setigerous pore on elytral interval 3 close to elytral base, very near level of scutellar apex ( Figs 4A, 4B, 4C View FIGURE 4 ); mandibles strongly widened, semicircular in form ( Fig. 2B View FIGURE 2 ); mentum with apex of epilobes not exceeding lateral lobes ( Fig. 3B View FIGURE 3 ); antennomere 2 with two long setae near apex, accessory setae absent or very short ( Figs 1C, 1D View FIGURE 1 ); male mesotarsomere 1 with adhesive setae ventral on apical half in most species................................................ subgen. Parena View in CoL s. str.
- First setigerous pore on elytral interval 3 distant from elytral base, far behind level of scutellar apex ( Figs 4D, 4E, 4F View FIGURE 4 ); mandibles moderately widened, rounded-triangular in form ( Figs 2C, 2D, 2E View FIGURE 2 ); mentum with apex of epilobes exceeding lateral lobes ( Figs. 3C, 3D View FIGURE 3 ); antennomere 2 with three or more setae of similar length near apex, in addition to a few short but distinct setae ( Fig. 1B View FIGURE 1 ); male mesotarsomere 1 without adhesive setae in most species............ subgen. Bothynoptera Schaum View in CoL
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Tribe |
Lebiini |
|
SubTribe |
Metallicina |
Parena Motschulsky, 1860
| Shi, Hongliang & Liang, Hongbin 2023 |
Euprymira
| Fairmaire 1901: 122 |
Prymira
| Fairmaire 1899: 76 |
Prymira stigmatica
| Fairmaire 1899 |
Umgenia
| Peringuey 1898: 324 |
Umgenia formidulosa
| Peringuey 1898 |
Metallica
| Chaudoir 1873 |
Phloeodromius
| Macleay 1871: 85 |
Parena
| Motschulsky 1860: 31 |