Chaetodontoplus poliourus, Randall & Rocha, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.5342290 |
|
publication LSID |
lsid:zoobank.org:pub:F9E677F0-4056-4B09-A8B1-88AD6F9F1288 |
|
persistent identifier |
https://treatment.plazi.org/id/B7BED97D-CAB9-4026-A1E5-A2A2C3D27B80 |
|
taxon LSID |
lsid:zoobank.org:act:B7BED97D-CAB9-4026-A1E5-A2A2C3D27B80 |
|
treatment provided by |
Diego |
|
scientific name |
Chaetodontoplus poliourus |
| status |
sp. nov. |
Chaetodontoplus poliourus View in CoL , new species
( Figs. 2 View Fig , 4–7 View Fig ; Tables 1, 2)
Chaetodontoplus sp. 1 Kuiter, 1992: 109 , Fig. D ( Flores).
Chaetodontoplus sp. Randall, 1998: 246 , Fig. 42 (Halmahera).
Chaetodontoplus mesoleucus View in CoL (non Bloch) Halstead, 2000: 135, upper Fig. ( Papua New Guinea and Solomon Islands).
Chaetodontoplus View in CoL cf mesoleucus Debelius et al., 2003: 132 View in CoL , Figs. A–E (Bali to Flores, Papua New Guinea, Solomon Islands, Palau).
Material examined. – Holotype: BPBM 39031 About BPBM , male, 81.5 mm SL, Papua New Guinea, New Britain, Kimbe Bay, first reef off Walindi Plantation , 6–10 m, spear, J. L. Earle, 19 Jul.2002.
Paratypes: USNM 150535 About USNM , 54.5 mm SL, Solomon Islands, Florida Islands , W. M. Chapman & H. Cheyne, dynamite, 4 May 1944 ; USNM 169824 About USNM , 76.0 mm SL, same data as preceding ; USNM 169785 About USNM , 2 ex., 64.0–77.0 mm SL, Solomon Islands, New Georgia, outer reef of Wana-Wana Island and Blacketts Strait, dynamite, W. M. Chapman & H. Cheyne, 25 Jun.1944 ; BPBM 6833 About BPBM , 85.0 mm SL, Palau, limestone islet east of Koror, coral reef, 6 m, spear, J. E. Randall, 1 Jun.1968 ; BPBM 6834 About BPBM , 2 ex., 81.5–92.0 mm SL, Palau, Ngargol Island , west end, coral bottom, 6–12 m, spear, J. E. Randall, 8 Jun.1968 ; USNM 209956 About USNM , 81.0 mm SL, Indonesia, Molucca Islands, Saparua, off Kampungmahu , isolated coral patch in 4 m, surrounded by calcareous matrix in 10 m, rotenone , V. G. Springer & M. F. Gomon, 18 Jan.1973 ; AMS I.19881-001, 90 mm SL, Solomon Islands, Sandfly Passage, Biki Island , coral reef, 20 m, spear, B. Goldman, 24 Jul.1973 ; USNM 245400 About USNM , 88.0 mm SL, Indonesia, New Guinea, Papua, Batanta Island , Marchesa Bay , Hawaii Islet , 0°49.8’S 130'56.8"E, 0–6 m, rotenone , RV Alpha Helix, B. B. Collette, 2 Jul.1979 ; BPBM 32217 About BPBM , 4 ex., 56.0– 80.5 mm SL, Indonesia, Flores, off Pertamina oil storage site, Waipare Reef , 8°37'46"S 122°16'35"E, 21 m, rotenone and spear, J. E. Randall GoogleMaps , R. H. Kuiter , & L. C. Reynolds, 19 Sep.1987 ; WAM P.33068- 001, 72.0 mm SL, same data as preceding ; BPBM 32239 About BPBM , 2 ex., 60.0–76.0 mm SL, Flores, Wodong Reef , 8°36'10"S 122°25'30"E, 20 m, quinaldine and spear, J. E. Randall, 20 Sep.1987 GoogleMaps ; MZB 17171, 79.5 mm SL, same data as preceding GoogleMaps ; BPBM 32580 About BPBM , 3 ex.: 76.0–88.0 mm SL, Papua New Guinea, Madang Province, Kranket Island , inner lagoon, live coral bottom, 3–4 m, spear, J. E. Randall, 12 Nov.1987 ; BPBM 32427 About BPBM , 2 ex., 42.0–71.0 mm SL, Madang, off Christiansen Research Institute, spear, P. L. Colin, late 1987 ; ROM 77374, 75.0 mm SL, Palau, Koror, SSE of Tlutkaraguis Island, off W coast of Ngerubktabel Island , reef between small and larger island, 7°17'11.7"N 134°25'34.3"E, 6.1–14.3 m, rotenone GoogleMaps , R. Winterbottom , W. Holleman, B. Hubley, D. Winterbottom & A. Bauman, 26 May 2004 ; ROM 84401, 77.5 mm SL, same data as holotype ; BPBM 40963 About BPBM , 2 ex., 57.0–107.0 mm SL, Palau, Koror, inland channel, 8 m, spear, L. A. Rocha, B. W. Bowen, & M . T. Craig , 23 Oct.2006 ; CAS 227415, 109 mm, MNHN 2009-002 About MNHN , 97.5 mm , NSMT-P 92943 , 96.0 mm SL, same data as BPBM 40963 ; USNM 394206 About USNM , 89.5 mm SL, Southwest Islands of Palau, Helen Reef , east side of lagoon opposite entry channel, just inside of wreck on reef top, 02°51'48"N 131°48'06"E, hard corals, lettuce corals, algae on rubble, and sand, 7–23 m, spear, M. W. Westneat & J GoogleMaps . T. Williams , 16 Sep.2008 ; FMNH 118117 About FMNH , 89.4 mm SL, same data as preceding .
Diagnosis. – Dorsal rays XII,17 (rarely 16 or 18); anal rays III,16 or 17; pectoral rays 15–17 (usually 16); scales small, about 80 in longitudinal series; dorsal series of pored lateralline scales 29–35; gill rakers 4 + 12; supraneural bones 2; body depth 1.70–1.85 in SL; head length 3.15–3.3 in SL; fourth to sixth dorsal spines longest, 1.1–1.3 in HL; colour in alcohol: head and anterior body pale yellowish grey to an approximate oblique demarcation connecting base of second to third dorsal spine to anus, gradually changing posteriorly in an intermediate zone containing pectoral fin to one of irregular longitudinal lines on dark brown; a dark brown bar from nape, broadening as it passes through eye, then curving and narrowing as it ends shortly before origin of pelvic fins; front of lips brown; dorsal and anal fins coloured as adjacent body; caudal fin abruptly pale grey; paired fins pale yellowish; colour in life: dark brown posteriorly with white dots, which merge anteriorly to form narrow irregular white lines; dark brown of body progressively lighter anteriorly, becoming pale grey at demarcation, then gradually changing to pale yellow on head; ocular bar black, narrowly edged in bluish white; snout and chest yellow, the front of lips blue, this colour sometimes continuing as a narrow triangle medially on front of snout; first three to four dorsal spines and membranes yellow, the rest of fin dark brown with rows of white dots paralleling rays on about basal three-fourths of fin; a narrow white or blue margin on soft portion of fin; anal fin similar, but not yellow anteriorly; caudal fin grey with a narrow yellow posterior border; pectoral fins pale grey; pelvic fins bright yellow.
Description. – Dorsal rays XII,17 (one of 23 paratypes with 16, and one with 18); anal rays III,17 (16 or 17); all dorsal and anal rays branched, the last to base; pectoral rays 16 (15–17, usually 16), the upper two and lowermost unbranched; pelvic rays I,5; principal caudal rays 17, the upper and lower unbranched; upper procurrent caudal rays 4, the most posterior segmented; lower procurrent caudal rays 3, the most posterior segmented; longitudinal scale series about 80; pored lateral-line scales 32 + 7 (29–35 + 7–9); gill rakers 4 + 12; pseudobranchial filaments 21 (14–23); branchiostegal rays 6; vertebrae 10 + 14; supraneural bones 2.
Body deep, the depth 1.7 (1.7–1.85) in SL, and compressed, the width 3.4 (3.05–3.4) in depth; head length 3.25 (3.15–3.3) in SL; dorsal profile of head forming an angle of about 60°, with a slight concavity above eye and a slight convexity before dorsal fin; snout short, the length 3.0 (2.75–3.1) in HL; orbit diameter 3.55 (3.1–3.95) in HL; interorbital width 3.6 (3.35–4.0) in HL; caudal-peduncle depth 2.55 (2.5–2.95) in HL; caudal-peduncle length 6.05 (4.7–6.1) in HL.
Mouth small, the maxilla reaching to below anterior nostril, and strongly oblique when fully closed, forming an angle of about 60° to horizontal axis of head and body; lower jaw strongly projecting; jaws very protrusible, the angle of mouth reduced to about 20° when jaws fully extended; lips broad, the median depth of upper lip about one-half orbit diameter; teeth in jaws in four rows, the inner rows progressively shorter; teeth close-set, long and slender, about twice as wide as thick, the tips slightly incurved, expanded and tricuspid; central cusp of teeth much the largest and strongly pointed; upper jaw of holotype with 38 teeth in outer row, and lower jaw with 40 (largest paratype with 34 upper teeth and 40 lower teeth); no teeth on palate; tongue short and rounded, set far back in mouth; gill membranes narrowly attached to isthmus; gill rakers short, about onesixth length of gill filaments.
Anterior nostril a short fleshy tubule with small opening a pupil diameter before centre of eye; posterior nostril a narrow elliptical aperture directly dorsoposterior to anterior nostril; no fleshy papillae midventrally on head.
A strong spine at corner of preopercle, its length 3.0 (2.6– 5.05) in HL; posterior margin of preopercle with 22 (20-38), small, unevenly spaced serrae, some as tiny nodules; lower margin of preopercle with 10 small serrae on one side of holotype and 14 on other (3–15 in paratypes); margin of subopercle with 1 (0–12) small serrae; preorbital with 1 (0–5) small serrae.
Dorsal part of lateral line strongly arched to middle of body, then curving downward to end near rear base of dorsal fin; separate midlateral part of lateral line on caudal peduncle, extending about a peduncle length anteriorly; scales on body not in regular rows, coarsely ctenoid, with up to 23 cteni, continuing as ridges across exposed part of scales; many scales on body with auxiliary scales (also ctenoid); scales smaller on head, progressively smaller anteriorly; scales extending out on dorsal and anal fins as rows of narrow oblique ridges, the scales progressively smaller distally; no scales on first two dorsal spines and membranes and about outer half of next two spines and membranes (naked part of fin not pigmented, in contrast to very dark remaining part of fin); caudal fin densely covered with very small scales; rays of pectoral fins with a row of close-set, quadrangular scales, only those basally on rays with a few cteni; pelvic fins with small ctenoid scales on rays.
Origin of dorsal fin above first lateral-line scale, the predorsal length 2.8 (2.7–2.9) in SL; first dorsal spine 2.5 (2.35–3.0) in HL; fourth to sixth dorsal spine longest, 1.2 (1.15–1.3) in HL; first dorsal soft ray longest, 1.35 (1.2–1.45) in HL; origin of anal fin below base of eleventh dorsal spine, the preanal length 1.55 in SL; first anal spine 2.0 (1.85–2.4) in HL; third anal spine longest, 1.35 (1.2–1.45) in HL; first anal soft ray longest, 1.3 (1.2–1.45) in HL; third to fifth pectoral rays longest, 1.4 (1.3–1.55) in HL; origin of pelvic fins below midbase of pectoral fins, the prepelvic length 2.75 (2.65–2.75); pelvic spine 1.35 (1.3–1.5) in HL; first pelvic soft ray longest, reaching posterior to anus, 1.2 (1.05–1.25) in HL.
Colour of holotype in alcohol: head and anterior body pale yellowish grey to an approximate oblique demarcation connecting base of second to third dorsal spines to anus, then gradually changing posteriorly in an intermediate zone containing pectoral fin to a pattern of irregular longitudinal pale lines on dark brown; a dark brown bar from nape, broadening as it passes through eye, then curving and narrowing as it ends shortly before origin of pelvic fins; front of lips dark brown, the dark pigment continuing medially on front of snout, forming a narrow triangle when viewed from the front, the apex above nostrils; dorsal and anal fins dark brown as adjacent body except pale yellowish anterior part of dorsal fin before an oblique demarcation from base of third dorsal spine to tip of fifth dorsal spine; caudal fin abruptly pale yellowish grey; paired fins pale yellowish, the membranes translucent.
Colour of holotype when fresh as in Figure 6 View Fig . Figures 4 View Fig and 5 are underwater photographs of other individuals from the type locality of Kimbe Bay, New Britain. Figure 5 shows the blue pattern of the lips and snout, as well as the variant with a bright blue margin on the soft portion of the dorsal and anal fins (bluish white on holotype).
Etymology. – We have selected the species name poliourus from the Greek meaning grey tail for its most distinguishing colour feature, the predominantly grey caudal fin.
Genetics. – As mentioned, our earlier efforts to distinguish this species morphologically from the yellow-tailed Chaetodontoplus mesoleucus were not successful, so we decided to check if a molecular difference could be determined. Tissue samples of the new species were obtained from type specimens from New Britain, Indonesia (Raja Ampat) and Palau, and from C. mesoleucus also from Indonesia (collected at the same date and locality as C. poliourus ) and from the Philippines (imported for the aquarium fish trade).
The genetic analysis revealed a very large difference between the species in both mitochondrial (mt) and nuclear DNA (nDNA). For the mtDNA, a 757 base pair fragment of the cytochrome b gene was obtained. There were 46 diagnostic mutations, corresponding to a 6.07% uncorrected sequence divergence between species. This divergence is equal to or greater than that observed between pairs of closely related sister species of angelfishes ( Bellwood et al., 2004), wrasses ( Rocha, 2004), butterflyfishes (Fessler & Westneat, 2007), and grunts ( Rocha et al, 2008). The nDNA analysis resulted in a 532 base pair segment of the S7 intron in C. mesoleucus and a 507 base pair segment of the same region in C. poliourus . The 25 base pair gap corresponds to an insertion or deletion (indel) between positions 220 and 245. This consistent difference in nDNA indicates that there is no ongoing hybridization between the two species.
Remarks. – While taking meristic data on additional specimens of the two species of Chaetodontoplus , we found that C. poliourus has modally 16 pectoral-fin rays, compared to 17 for C. mesoleucus , and a lower average number of anal-fin rays (Table 2).
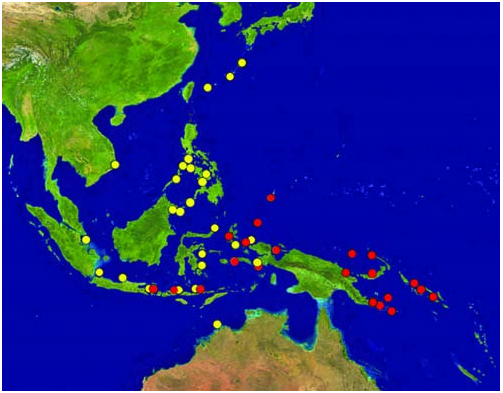
The distribution of the two species of Chaetodontoplus ( Fig. 10 View Fig ) was determined mainly from specimens examined by the authors, specimens in the California Academy of Sciences examined for the authors by David Catania and Richard L. Pyle, underwater photographs taken by the first author ( Figs. 8 View Fig , 9), and the following localities for C. poliourus provided by Gerald R. Allen from his field work in Indonesia and Papua New Guinea: Cenderwasihi Bay, Milne Bay, D’Entrecasteaux Islands, Woodlark Island, Louisade Archipelago, Manus Island, and the northern tip of New Ireland. He added the following localities for the Solomon Islands: Choiseul, Shortland Islands, Russell Islands, and Guadalcanal. He found both species at the islands of Misool and Kofiau off the western end of New Guinea where C. mesoleucus was by far the most common. Conversely, C. poliourus was relatively common along the west side of Halmahera, but only one individual of C. mesoleucus was observed (at Widi Islands, 0°33.4'S, 128°20.6'E). Other Allen records for C. mesoleucus include Banggai Island off eastern Sulawesi and the Kimberly coast of Western Australia at Cape Bougainville. John L. Earle (pers. comm.) observed both species at Raja Ampat and at Komodo. He wrote, “They are often observed on the same dive, however not in the same micro-habitat, and never as mixed species pairs”.
Masuda & Kobayashi (1994: 206, Fig. 4 View Fig ) illustrated a juvenile of C. mesoleucus , 25 mm total length, from a photo taken at the Yaeyama Islands, Japan. The northernmost record for C. mesoleucus is that of Masuda et al. (1975: 314, pl. 12a) from the Amami-Ô-Shima Islands. Another example of live colouration in Japan may be seen in Okamura & Amaoka (1997: 404, middle right fig.).
Allen et al. (1998: 67) reported that Chaetodotoplus mesoleucus (they illustrated both mesoleucus and poliourus as yellow-tailed and grey-tailed forms, respectively) feeds on sponges, tunicates, and algae.
Taxonomic notes. – We were concerned that Chaetodon atratus Gronow in Gray (1854), with a locality of India, might be an earlier name for Chaetodontoplus poliourus . Fraser- Brunner (1933: 550) corrected the locality to Singapore, but without explanation. We obtained a photograph of the dried skin of the holotype from James Maclaine of the Natural History Museum in London. Only the first dorsal spine and part of the second is pale, so we confidently identify this taxon as C. mesoleucus .
There was also a possibility that Holacanthus bicolor var. oahuensis Borodin, 1930 , placed in the synonymy of C. mesoleucus by Fraser-Brunner, might predate C. poliourus . Borodin’s record of this species from Oah’u is obviously a locality error, as are three other of his records of fishes for the Hawaiian Islands ( Mundy, 2005: 406). His holotype, formerly in the Vanderbilt Marine Museum and now in the American Museum of Natural History, was examined by Richard L. Pyle (pers. comm.), who identified it as C. mesoleucus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Chaetodontoplus poliourus
| Randall, John E. & Rocha, Luiz A. 2009 |
Chaetodontoplus
| Debelius, H 2003: 132 |
Chaetodontoplus mesoleucus
| Halstead, B 2000: 135 |
Chaetodontoplus sp. Randall, 1998: 246
| Randall, J 1998: 246 |
Chaetodontoplus sp. 1 Kuiter, 1992: 109
| Kuiter, R 1992: 109 |