Litoria spaldingi ( Hosmer, 1964 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4933.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:C52F83E2-2D42-4964-8AC0-9503085DD625 |
|
DOI |
https://doi.org/10.5281/zenodo.4560005 |
|
persistent identifier |
https://treatment.plazi.org/id/03888794-FFF7-4A31-FF04-F9FAFB05F99B |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria spaldingi ( Hosmer, 1964 ) |
| status |
|
Litoria spaldingi ( Hosmer, 1964)
Northern Creek Frog
( Figs 6 View FIGURE 6 , 9 View FIGURE 9 , 10 View FIGURE 10 )
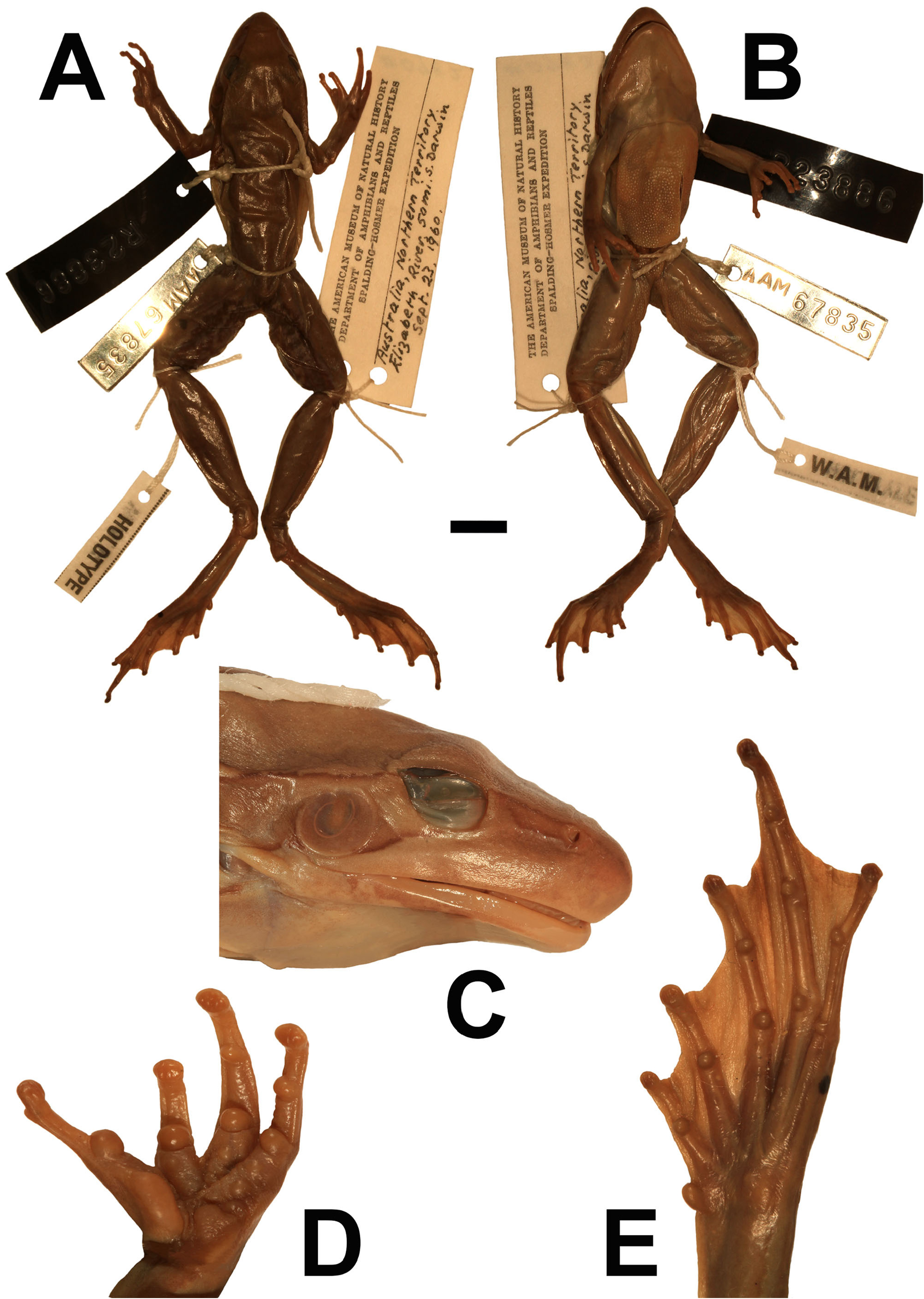
Holotype. An adult female, WAM R 23886 View Materials (formerly AMNH No. 67835), collected at Elizabeth River, 50 miles south of Darwin , Northern Territory, on 23 September 1960 by William Hosmer.
Material examined. Specimen details are listed in the Appendix I.
Type measurements. Hosmer (1964) presented a detailed description of the holotype ( Fig. 9 View FIGURE 9 ), but few measurements. Measurements (mm): SVL = 51.3, HL 19.4, HW = 18.1, TD = 3.9, ED = 4.7, EN = 5.3, IOD = 8.9, IND = 4.9, FLL = 12.6, Fin3L = 9.9, TL = 31.8, Toe4L = 27.3.
Diagnosis. Morphologically shares the external features of L. watjulumensis (see above), but is distinguished from that species by divergence in mitochondrial and nuclear DNA markers ( Figs 2 View FIGURE 2 , 3 View FIGURE 3 ). From a genetic perspective, apomorphic nucleotide states at 12 and three sites in the mitochondrial ND4 and the nuclear PTPN12 genes respectively reliably diagnose L. spaldingi from L. watjulumensis ( Table 5 View TABLE 5 ).
Description including variation. Assessment of morphological variation is based on 16 females and 31 males ( Table 2 View TABLE 2 ). SVL females mean = 47 mm, males mean = 38 mm. Head slightly longer than broad (HL/HW 1.17), and approximately one third snout to vent length (HL/SVL 0.39). Snout prominent, pointed when viewed from above blunt when viewed in profile. Nostrils more lateral than superior, closer to tip of snout than to eye. Distance between eye and naris equal to internarial span (EN/IND 1.03). Canthus rostralis well defined and straight. Eye size moderate, its diameter equivalent eye to naris distance. Pupil horizontal when constricted. Tympanum distinct, circular, length slightly greater than half eye diameter (TD/ED 0.62). Supratympanic fold absent or poorly developed. Vomerine teeth short straight plates bridging the gap between the choanae. Tongue approximately rectangular.
Fingers long, slender, unwebbed. Subarticular and palmar tubercles prominent. Terminal discs not extending beyond lateral extremities of penultimate phalanx. Dark brown nuptial pad on upper and inner surface of the proximal half of the first finger. Fingers in order of length 3>4>1>2. Hindlimb length moderate (TL/SVL 0.67). Toes in order of length 4>5=3>2>1. Webbing reaches base of second most distal phalanx on toe 4 and penultimate phalanx on other toes. Subarticular tubercles prominent. Small oval inner metatarsal tubercle present. Terminal toe discs not wider than toes ( Fig. 9 View FIGURE 9 ).
Dorsum either smooth or with low and infrequent tubercles sometimes forming lines parallel to long axis of the body. Limbs with low tubercules, sometimes smooth. Abdomen, undersurface of thighs, and lateral aspect of body mildly granular. Pectoral fold absent in a majority of specimens and when present is indistinct. Small vocal sac present. Vocal slit at base of mandible aligned along posterior-anterior axis, approximately one quarter length of mandible.
Colour in life. Dorsum pale to light brown, mottled with dark brown, imparting an overall impression of a more-or-less uniformly light brown or dark brown animal ( Fig. 10 View FIGURE 10 ). In calling males yellow is suffused along the dorso-lateral edge of the dorsum from the eye to the groin and onto the lower back, onto upper forelimb and throat. Upper surfaces of limbs with same colour as dorsum, rarely with dark flecks. Face pale to light brown with dark brown stripe beginning at tip of snout and confined to upper edge of canthus rostralis, becoming more prominent after the nostril, continuing past the eye to mid-body with width same as eye and encompassing tympanum. In calling males, face often suffused with yellow. In some individuals, dark blotches extend from dorsal end of mid-lateral stripe to groin. Upper and lower lips either immaculate or have prominent light brown or white and dark brown patching. Dorsal surface of head often lighter than rest of dorsum in heavily patterned animals.
Rear of thighs mostly dark brown, often same colour as facial stripe with numerous yellow and light brown circular to linear shaped spots and patches occupying between 20–50% of rear of thigh ( Fig. 6 View FIGURE 6 ). Yellow marks more frequent closer to knee, light brown marks dominate closer to vent. Vent same colour as surrounding dorsal colours. Fore of thighs either unpatterned or with same colour and pattern as rear of thigh but separated by intrusion of dorsal colour along dorsal surface of thigh. Groin with dark brown and yellow patching. Fore of lower limbs edged with dark brown in some individuals. Outer margin of foot well demarked by distinct border between lighter upper surface and dark brown plantar surface.
Abdomen plain white, lower abdomen sometimes suffused with light brown flecking, otherwise immaculate. Throat white, sometimes with small indistinct dark mottling, otherwise unpigmented. Upper iris red margined with a distinct white band that appears continuous with upper margin of head stripe, lower iris same dark brown as head stripe.
Distribution and habitat. Occurs in the IBRA regions of the Daly Basin, Darwin Coastal, Pine Creek, Arnhem Coast, Arnhem Plateau, Central Arnhem, Gulf Coastal, Gulf Fall and Uplands, Mount Isa Inlier, and Gulf Plains ( Fig. 1 View FIGURE 1 ). The most western locality is in the Litchfield National Park, NT (NTM R21723), and the most eastern and southern is the East Leichhardt River near the East Leichhardt Dam, Qld (SAMA R63646). One voucher, NTM R31013, a poorly preserved juvenile from the Murranji Stock Route, is the only record from the Sturt Plain bioregion. In Kakadu National Park and in the vicinity of Darwin, L. spaldingi is associated with riparian forests ( Woinarski & Gambold 1992, Reynolds et al. 2010).
Breeding biology. Tyler et al. (1983) provide a detailed account [as L. wotjulumensis ] of the breeding biology, variation in egg and larval morphology and development of frogs from Magela Creek, a tributary of the East Alliga-tor River on the western periphery of the Arnhem Land plateau. Males called from the edge of water from October to March and breeding occurred early in the wet season. Spawn were found in temporary pools on sandy or gravelly soils.
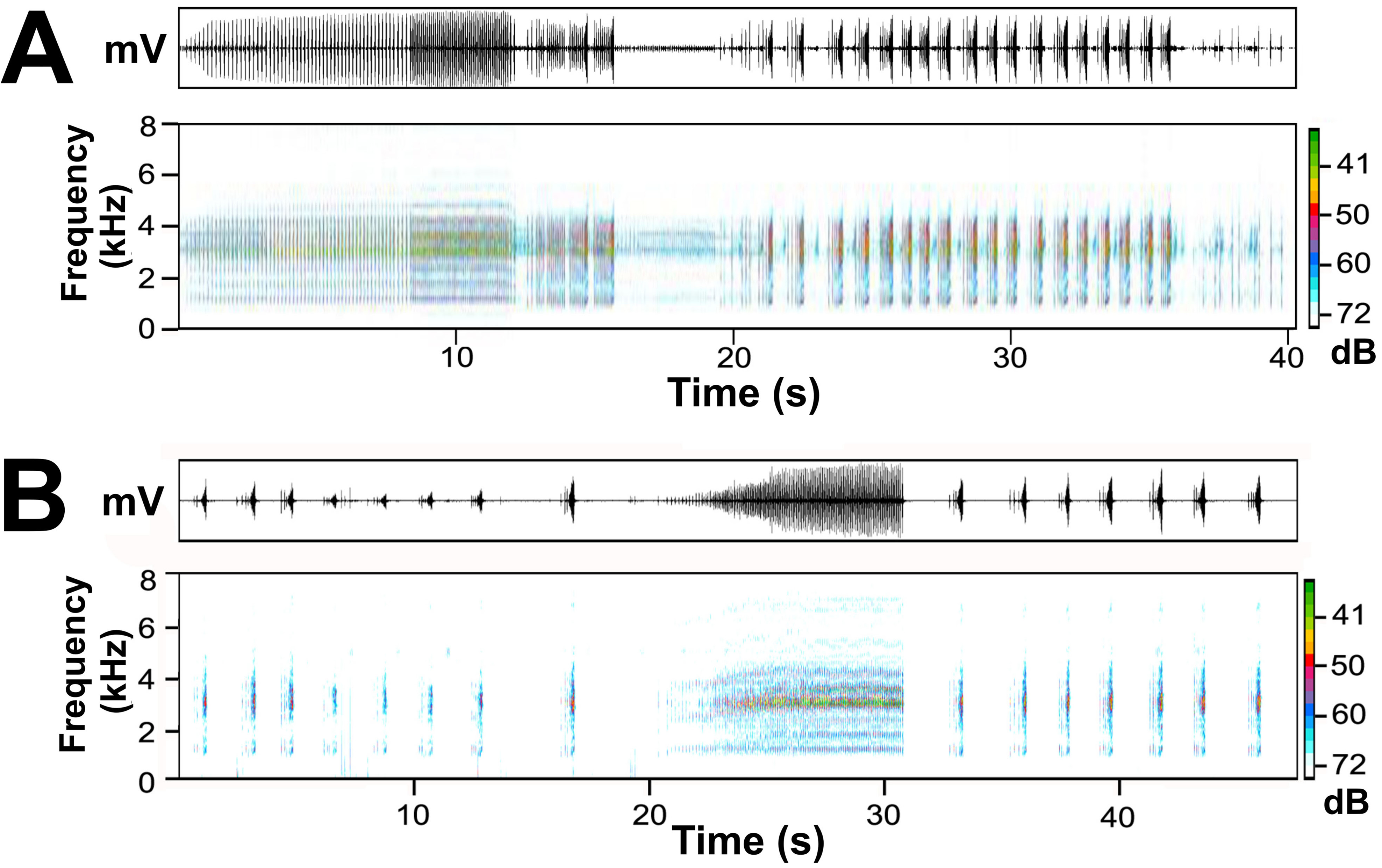
Advertisement call. Litoria spaldingi produces calls in long series that can last at least one minute (the maximum recording time of the FrogID app). Calls are similar to those of L. watjulumensis and exhibit similar levels of complexity and variation, but available recordings are dominated by short calls and long calls. These are similar to the short calls and long calls described above for L. watjulumensis . Clucks (see description of L. watjulumensis calls) were rarely recorded, but this may reflect a lack of social interactions between males on recorded sequences of L. spaldingi . Although some audio files contained only long or short calls, it is not possible to exclude the possibility that both call types were present before or after the limited segments on these audio files, because both call types occurred on several other recordings ( Fig. 8B View FIGURE 8 ). Of 164 calls scored for call type, 135 (82%) are short calls and 29 (18%) are long calls. This difference is not attributable to sampling bias among contributors to FrogID because a similar ratio was observed on individual audio files containing both call types (e.g. Fig. 8B View FIGURE 8 ).
Short calls are produced at intervals of 0.4–12.4 seconds (mean = 1.68, SD = 1.53, n = 96), and have a length of 0.29–0.69 seconds (mean = 0.47, SD = 0.07, n = 74). They typically comprise two discrete components: the first is a series of introductory notes produced at 0.05– 0.17 s intervals (mean = 0.09, SD = 0.03, n = 28). Only one of 78 short calls had no introductory notes (1.3%), four had one introductory note (5.1%), 14 had two introductory notes (18%), 55 had three introductory notes (70.5%) and four calls had four introductory notes (5.1%). Introductory notes are pulsatile, but amplitude modulation does not approach 100% except in rare cases ( Fig. 8B View FIGURE 8 ). The second component of short calls is a rapidly-repeated series of ~7–12 terminal notes produced at much shorter intervals than short notes (approximately 0.006 – 0.010 s, but inter-note interval was difficult to measure accurately in most calls). A conspicuous feature of the terminal part of short calls is the rapid reduction in inter-note intervals across the course of this component. To the human ear short calls sound like a series of sharp clicks, followed by a short buzz during which note repetition rate increases rapidly. Terminal notes are also pulsatile, and there are some examples of 100% amplitude modulation producing distinct pulses. Amplitude of introductory notes is variable ( Fig. 8B View FIGURE 8 ), but in terminal components amplitude starts very low and increases gradually, reaching maximum amplitude at the end of the call.
Long calls are at least five times the duration of short calls (4.2– 12.2 s; mean = 8.2, SD = 2.2, n = 23), and lack introductory notes. Twenty-three long calls contained 56–129 notes (mean = 94.1, SD = 24.8) produced at a note repetition rate of 10.9–14.4 notes/s (mean = 12.9, SD = 1.1, n = 12). Amplitude is low at the start and increases gradually, typically reaching maximum amplitude approximately half to one third of the way through the call, then remains steady before dropping abruptly at the end of the call ( Fig. 8B View FIGURE 8 ). However, one long call did not reach maximum amplitude until approximately three quarters of the way through the call. Notes are pulsatile but rarely achieve 100% amplitude modulation, although this is variable among calls. In some long calls each note is divided into two components: a pulsatile introductory component followed by 1–2 discrete pulses. Note repetition rate increases rapidly during the first ~20–25% of the call ( Fig. 8B View FIGURE 8 ), and the call sounds to the human ear like a series of sharp clucks repeated increasingly rapidly and grading into a long, harsh chattering sound. Sixteen of 29 long calls terminate with 1–3 discrete notes reminiscent of short calls, or of the second component of short calls, i.e. a short ‘buzz’.
Frequency is broadly distributed in both short and long calls ( Fig. 8B View FIGURE 8 ) so the calls have a harsh quality. Dominant frequency of both call types is similar: 2490–3372 kHz (n = 19) in short calls and 2688–3007 kHz (n = 11) in long calls, but one long call had a dominant frequency that was carried by a poorly defined lower harmonic at 1540 kHz.
Hoskin et al. (2015) present audio files of the advertisement calls of several males from Berry Springs and western Arnhemland.
Comparisons between L. watjulumensis and L. spaldingi calls. The calls of L. watjulumensis and L. spaldingi are similar, and notable for their complexity. For convenience we have recognised three discrete call types: clucks, short calls and long calls. However, each of these call types exhibits variation in note structure within and between calls, and observations in the field indicate that some structural features of calls are adjusted by males according to social factors ( Tyler & Doughty 2009).
Given the unusually high variation in structural features of calls, and small sample sizes available for analysis (calls from just one male L. watjulumensis were available), robust comparisons between calls of L. watjulumensis and L. spaldingi were not feasible and consequently taxonomic implications of most differences observed are uncertain. One exception may be the striking difference in patterns of note rate adjustment during long calls. In long calls of L. watjulumensis , note repetition rate is relatively uniform during the first half of the call, then doubles abruptly (between two consecutive notes) and remains at the higher rate (with rare longer intervals between several notes) for the remainder of the call. In contrast, note rate in long calls of L. spaldingi gradually increase from the start of the call. Differences between these patterns are evident in Fig. 8 View FIGURE 8 . However, further studies are required to determine whether these patterns are consistent, and if so whether this feature of the species’ advertisement calls may be a useful diagnostic character in the field.
Remarks. In his 1963 description of H. spaldingi, Hosmer cited Copland’s (1957) monograph but did not mention H. latopalmata watjulumensis , despite the latter occurring approximately 400 km WSW from the nearest record of his new species. Hosmer mentions examining 76 specimens of L. latopalmata , but provides no registration numbers or indication of the collection they were from (presumably AMNH). Based on their small adult body size, these were likely L. latopalmata from the eastern coast.
Subsequently Tyler’s description of L. (Hyla) coplandi Tyler 1968 , is the first time that adequate geographical representative material was used to assess morphological variation in L. watjulumensis sensu lato.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Litoria spaldingi ( Hosmer, 1964 )
| Donnellan, Stephen C., Catalano, Sarah R., Pederson, Stephen, Mitchell, Kieren J., Suhendran, Aidan, Price, Luke C., Doughty, Paul & Richards, Stephen J. 2021 |
Hyla spaldingi
| Hosmer 1964 |