Henneguya
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3887.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:B697D6BA-836B-44E2-A8D9-07661554FE59 |
|
DOI |
https://doi.org/10.5281/zenodo.5626252 |
|
persistent identifier |
https://treatment.plazi.org/id/038887C4-FFD4-FFCD-B3BB-F9D986744550 |
|
treatment provided by |
Plazi |
|
scientific name |
Henneguya |
| status |
|
Henneguya sp.
Type host: Sarpa salpa Linnaeus, 1758 goldline sea bream ( Perciformes : Sparidae )
Type localities: Mediterranean off Tunisia: Location 1: Gulf of Tunis (36°45’N, 10°15’E); Location 2: Bay of Bizerte (37°20’ N, 9°53’ E).
Site of infection: Mesenteric vessels
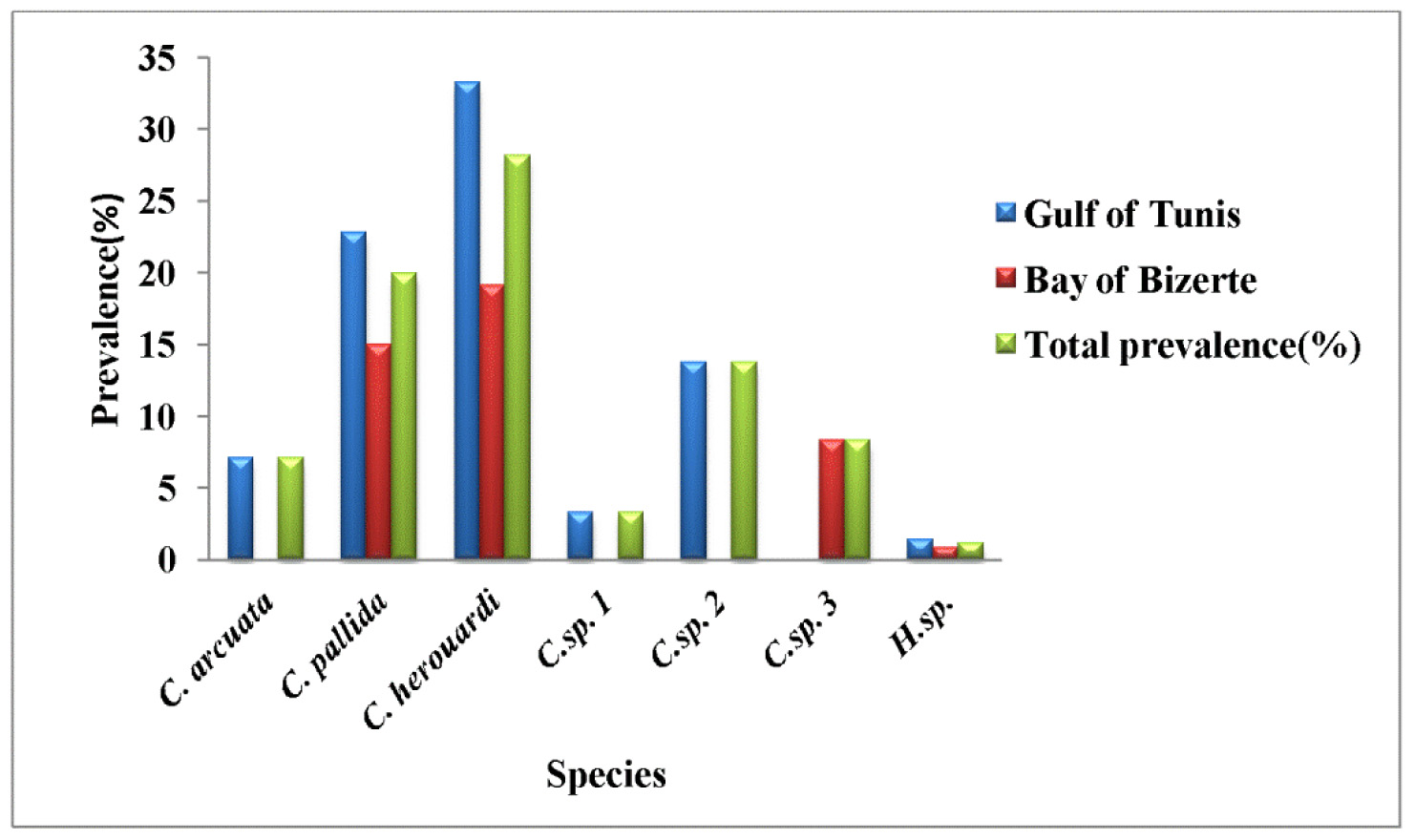
Prevalence: The overall prevalence is 1.2% (4/330) ( Fig. 9 View FIGURE 9 ). At location 1, the prevalence of infection is 1.4% (3/210) distributed as following, 03/2012: 0% (0/30); 04/2012: 10% (3/30); 05/2012: 0% (0/30); 06/2012: 0% (0/ 30); 07/2012: 0% (0/30); 08/2012: 0% (0/30); 05/2013: 0% (0/20); 06/2013: 0% (0/10). At location 2, the prevalence of infection is 0.8% (1/120) distributed as following, 03/2013: 0% (0/30); 04/2013: 0% (0/30); 05/2013: 3.3% (1/30); 06/2013: 0% (0/30) (see Table 4).
Mean intensity: 5 ± 2.5 cysts/infected fish ( Fig. 10 View FIGURE 10 ) (see Table 4).
Type-material: Digitized photos of syntype spores were deposited in the parasitological collection of the Museum National d’Histoire Naturelle ( MNHN), Paris, Coll. No. ZS 130.
Description
Vegetative stages. The parasite was found within mesenteric vessel of the host. Cysts were oval to round in shape and unequal in size with development asynchronous. They are measuring from 1.5 to 4 mm in diameter, filled with fluid containing a suspension of mature and immature spores which appear milky white by naked eye ( Fig. 7A View FIGURE 7 A – F ).
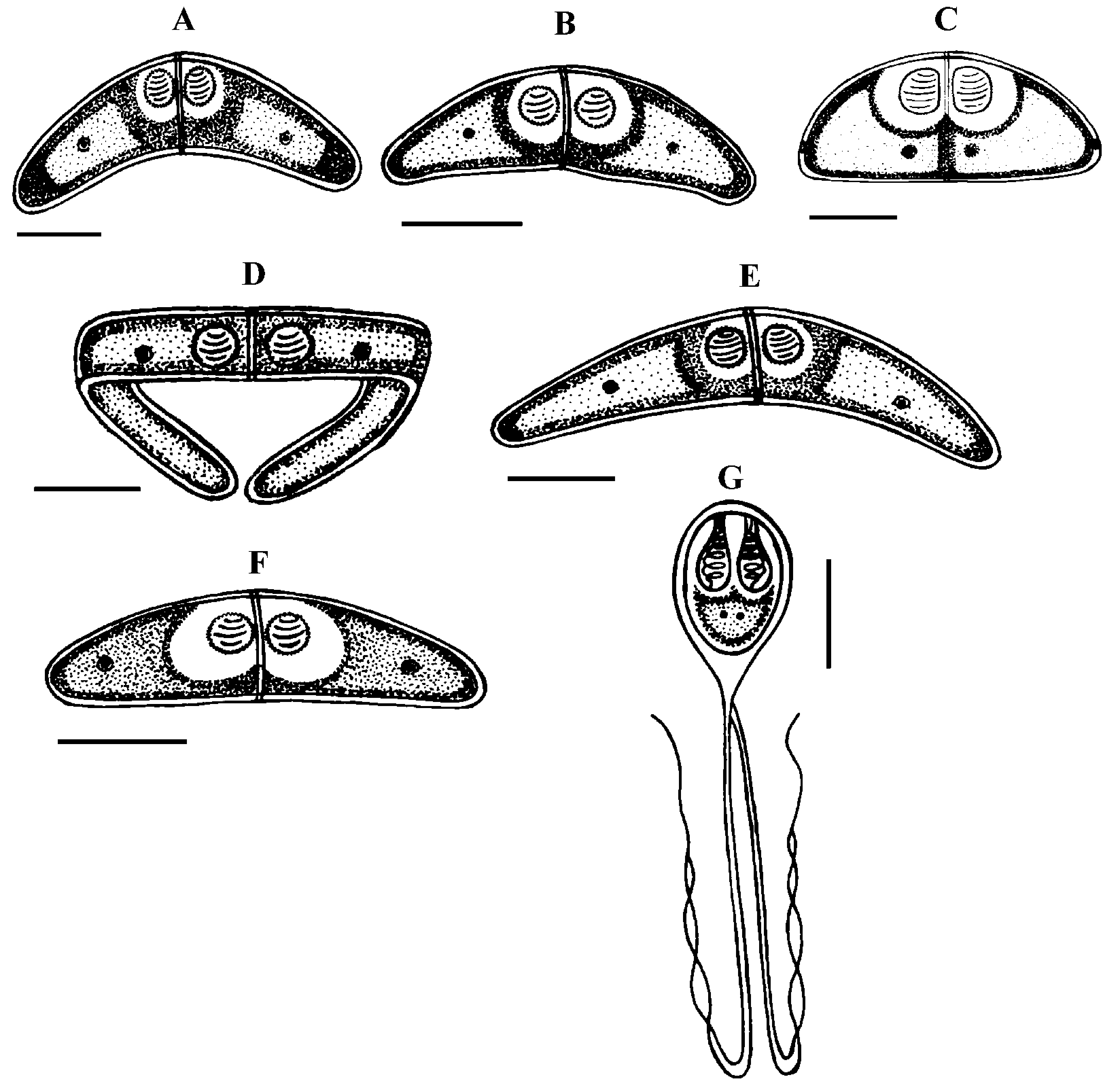
Spores (n = 30 fresh spores). Spores typical of the genus Henneguya . Mature spores were ovoid in side view with slightly attenuated posterior end ( Fig. 7C–G,J View FIGURE 7 A – F View FIGURE 7 G – K , 8 View FIGURE 8 G) and ellipsoidal in sutural view ( Fig. 7I View FIGURE 7 A – F View FIGURE 7 G – K ). Two shell valves smooth and equal in size with wide sutural ridge. Spore body measuring 15.26 ± 1.34 (12.6–16.2) µm in length, 9.94 ± 0.71 (9–10.8) µm in width and 8.6 ± 0.37 (8.2–9) µm in thickness. Two polar capsules were pyriform and equal in size, 5.55 ± 0.20 (5.4–5.78) Μm in length and 2.55 ± 0.1 (2.48–2.65) µm in width (n = 30). The polar filament coiled with five to six turns. Two capsulogenic nuclei located between the polar capsules ( Fig. 7F View FIGURE 7 A – F ). A binucleate sporoplasm situated directly behind two polar capsules, filling almost the entire spore cavity. Ten sutural markings were distinct and usually arranged all around the circumference of the spore and being more spaced in the posterior part than at the anterior one ( Figs. 7D–E View FIGURE 7 A – F , G View FIGURE 7 G – K ). Two caudal projections were filiform and long extending from posterior of spore with 49.32 ± 4.43 (42.3–55.8) µm in total length ( Figs. 7J–K View FIGURE 7 A – F View FIGURE 7 G – K , 8 View FIGURE 8 G). Often, the fine distal portion of the appendage wrapped around the thicker part ( Figs. 7F View FIGURE 7 A – F , J View FIGURE 7 G – K , 8 View FIGURE 8 G). Total length of spores was 64.58 ± 4.62 (58.5–70.2) Μm.
Taxonomic affinities
According to the scientific papers, only one histozoic species belong to genus of Hennguya Thélohan, 1892, H. neapolitana Parisi, 1912 has been described infecting the connective tissue of the renal tubule of kidney of S. salpa . The comparison between both species provides that current species differs from H. neapolitana not only by the organ host but also by the shape and the morphometric measurements (see Table 6). According to the study of Parisi (1912), H. neapolitana found in the connective tissue of the renal tubule of kidney of S. salpa with small cyst (40 to 50 µm Ø) while present species is found in the mesenteric vessels and forming a big white cysts (1-4 mm Ø) ( Fig. 7A View FIGURE 7 A – F ). Concerning the shape of the spores, H. neapolitana is more ovoid than the recent form and it polar capsules often cross each other while those of present finding are almost parallel to each other and to the sutural rim. Moreover, the spores of the current species are more longer than those of H. neapolitana in length and in width of the spore body and especially in the length of caudal projections (see Table 6). Among all the species of Henneguya found in the Mediterranean Sea, no species found in the mesentery vessels of its host. All the Henneguya spp. described from the sparids are found especially infecting the heart tissue (arterial bulb), the kidney or the gills. From these known species, the recent isolate shows some similar morphological appearances to H. mbourensis and H. yoffensis Kpatcha, Faye, Diebakate, Fall & Toguebaye, 1997 infecting the kidney of Dentex canariensis (Steindachner, 1881) and the gills and heart of P. caeruleostictus (Valenciennes, 1830) respectively from Senegal, H. pagri Yokoyama, Itoh & Tanaka, 2005 infecting the bulbus arteriosus of Pagrus major (Temminck & Schlegel, 1843) from Japan, H. mauritaniensis Khlifa, Miller, Adlard, Faye & Sasal, 2012 from the arterial bulb of Pagrus caeruleostictus off Mauritania and Henneguya sp. Bahri, Benhassine & Marques, 1996 found in the gills of Sparus aurata (Linnaeus, 1758) from Tunisia that was being the same species observed by Caffara, Marcer, Florio, Quaglio & Fioravanti, 2003 in the bulbus arteriosus and the gills of the same host from Italian fish farm (see Table 6). Although all these species are very similar in shape to current species, they each exhibit one or more distinguishing characteristics. Generally all these species are shorter in total length and have spores smaller in length and width compared to those of present form (see Table 6).
According to the synopsis of Henneguya ( Eiras 2002, Eiras & Adriano 2012), only 2 species of Henneguya found infecting the mesentery of their hosts H. visceralis Jakovska & Nigrelli, 1953 found in Electrophorus electricus (Linnaeus, 1766) from Brazil and H. schakletoni Brickle, Kalavati & MacKenzie, 2006 in Eleginops maclovinus (Valenciennes, 1830) from Off the Falkland Islands (see Table 6). The recent species differs from H. visceralis by having a larger spores with shorter polar capsules and a very long caudal appendages. Although, most of the morphometric measurements between present finding and H. schakletoni overlap, the spores of this latter are morphologically quite different. Furthermore, the current species has larger polar capsules and longer caudal projections than those of H. schakletoni . According to the paper of Brickle et al. (2006) the spores of H. schakletoni contained one large vacuole (2.0– 3.0 Ø) placed between the posterior ends of the polar capsules and the sporoplasm which is lacking at the present species. Moreover, the cysts of our species are much larger than those of H. schekletoni (1-4 mm vs 0.5-0.8 mm) and is, therefore, a distinct species. Another species H.
lateolabracis Yokoyama, Kawakami, Yasuda & Tanaka, 2003 found infecting the heart (bulb arteriosus) of the sea bass fish Lateolabrax sp. This species shows great superficial similarities in shape to our species. However, no measurement range overlap between both species (see Table 6). The recent isolate seems to have spores more bigger with longer caudal appendages compared to the spores of H. lateolabracis . Furthermore, the difference in site of infection confirms that both species are dissimilar. In light of these differences with closely related species, host organ and locality records, the myxosporean under study is considered as a different species and is reported by the first time infecting the sparid S. salpa in the Mediterranean Sea.
Ecological notes
In this study, Henneguya sp. found with very weak overall prevalence 1.2%. This myxosporean has a parasitic status as scarce species. In Gulf of Tunis, the infection was only observed in April with prevalence 10% and mean intensity 6 cysts per infected individual host whereas in Bay of Bizerte infection by this parasite was noted only in May with prevalence 3.3% and mean intensity 4 cysts per infected individual host (see Table 4).
Species Host(s) Locality Spore Polarcapsule PA(°)
SL ST PCL PCW
. arcuata Kalavati & Mackenzie Pagellus bogaraveo France ( Monaco), Italy 6.8 ± 0.9 36.2 ± 2.7 3.7 ± 0.7 3.0 ± 0.2 ND
1999) (6.0–9.0) (32.5–40.0) (2.5–5.0) (2.5–4.0)
Present study) Sarpa salpa Tunisia (Gulf of Tunis) 7.5 ± 0.4 35.6 ± 3.3 3.3 ± 0.4 3 ± 0.4 150.6 ± 4.2 (7–9) (30–40) (3–4) (2.5–3.5) (142–156)
. pallida Thélohan (1895) Boops View in CoL boops View in CoL France ( Monaco) 5 25–30 ND ND ND
Present study) Sarpa salpa View in CoL Tunisia (Gulf of Tunis and 7.32 ± 0.61 (6.5– 28 ± 1.5(26–30) 2.95 ± 0.47 2.92 ± 0.39 160.9± 4.6 Bay of Bizerte) 8) (2.5–3.6) (2.5–3.6) (154–170)
. herouardi Georgévitch (1916) Sarpa salpa View in CoL France ( Monaco) ND ND ND ND ND
Present study) Sarpa salpa View in CoL Tunisia (Gulf of Tunis and 10.5 ± 1.2 21.6 ± 1.6 3.91 ± 0.25 3.89 ± 0.27 172.5± 6.8 Bay of Bizerte) (8–12) (20–24) (3.5–4.5) (3.5–4.5) (165–180)
. sp. 1 (Present study) Sarpa salpa View in CoL Tunisia (Gulf of Tunis) 7.32± 0.52 67.98 ± 2.44 3.26 ± 0.23 3.26 ± 0.23 α =35.6 ± 12.3 (6.52–7.92) (64.9–70.1) (3–3.5) (3–3.5) (28–57)
. sp. 2 (Present study) Sarpa salpa View in CoL Tunisia (Gulf of Tunis) 9.73 ± 0.63 40.32 ± 3.83 4.2 ± 0.2 3.51 ± 0.39 150.2 ± 2.9 (9–10.5) (35–45) (4–4.5) (3–4) (146–155)
. sp. 3 (Present study) Sarpa salpa View in CoL Tunisia (Bay of Bizerte) 7.4 ± 0.8 30± 1.8 (28–33) 3 ± 0.41 3 ± 0.41 168.5 ± 4.2 (6.5–8.5) (2.5–3.5) (2.5–3.5) (162–172)
. diplodae Lubat et al. (1989) Diplodus annularis View in CoL Montenegro 6 (5–7) 20 (18–22) 2.25 2 ND
Katharios et al. (2007) Diplodus puntazzo View in CoL Greece 6.6 ± 0.5 24.0 ± 0.8 2.7 ± 0.2 2.7 ± 0.2 ND
. sparusaurati Sitja-Bobadilla et Sparus aurata View in CoL Spain 5.65±0.74 15.76 ± 1.01 2.79 ± 0.27 2.79 ± 0.27 ND
. (1995) (4.5–7.5) (14–17.5) (2.2–3.4) (2.2–3.4)
. puntazzi Alama-Bermejo et al. Diplodus puntazzo View in CoL Spain 9.2 ± 0.7 29 ± 2.9 4.1 ± 0.4 4 ± 0.4 166.2 ± 7.4
2011) (8.03–10.72) (23.83–34.5) (2.95–4.77) (2.9–4.6) (146.4–179.2)
. sp. Alama-Bermejo et al. Diplodus annularis View in CoL Spain 9.8 ± 0.8 28.8 ± 3.7 4.1 ± 0.6 4.1 ± 1.1 164.8 ± 2
2011) (7.1–13) (21.5–32.7) (3.2–5.2) (3.1–5.1) (147– 176.2)
. sp. 1 Alama-Bermejo et al. Sparus aurata View in CoL Spain 5 ± 0.5 17.2 ± 3.4 2.2 ± 0.4 2.1 ± 0.3 175.2 ± 4.1
2011) (3.9–5.6) (13.1–22.5) (1.6–2.7) (1.5–2.5) (166.9–179.9)
. sp. 2 Alama-Bermejo et al. Sparus aurata View in CoL Spain 9.9 ± 0.6 20 ± 2.1 3.8 ± 0.3 3.8 ± 0.4 169.2 ± 6.5
2011) (8.7–11.4) (16.7–24.7) (3.2–4.5) (3.2–4.5) (155.4–178.8)
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Henneguya
| Laamiri, Sayef 2014 |
diplodae
| Lubat et al. 1989 |
herouardi Georgévitch (1916)
| Georgevitch 1916 |
pallida Thélohan (1895)
| Thelohan 1895 |