Nectophrynoides viviparus ( Tornier, 1905 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.275339 |
|
DOI |
https://doi.org/10.5281/zenodo.5626178 |
|
persistent identifier |
https://treatment.plazi.org/id/0388DF54-FFC7-3A32-64B9-FD682D4D6B87 |
|
treatment provided by |
Plazi |
|
scientific name |
Nectophrynoides viviparus ( Tornier, 1905 ) |
| status |
|
Nectophrynoides viviparus ( Tornier, 1905) View in CoL
Pseudophryne vivipara Tornier, 1905 , Sitzungsber. Preuss. Akad. Wiss. Berlin, 39: 855.
Nectophrynoides werthi Nieden, 1910 View in CoL , Sitzungsber. Gess. naturf. Freunde Berlin, 10: 439.
Tornierobates vivipara Miranda-Ribeiro, 1926, Arq. Mus. Nac., Rio de Janeiro, 27: 19.
Nectophrynoides vivipara Noble, 1926 , Am. Mus. Novit., 212: 15. Barbour and Loveridge, 1928, Mem. Mus. Comp. Zool., 50: 191.
Nectophrynoides viviparus Perret, 1971 View in CoL , Ann. Fac. Sci. Cameroun, 6: 99.
Nectophrynoides viviparus Anon., 1996 View in CoL , Bull. Zool. Nomencl., 53: 229.
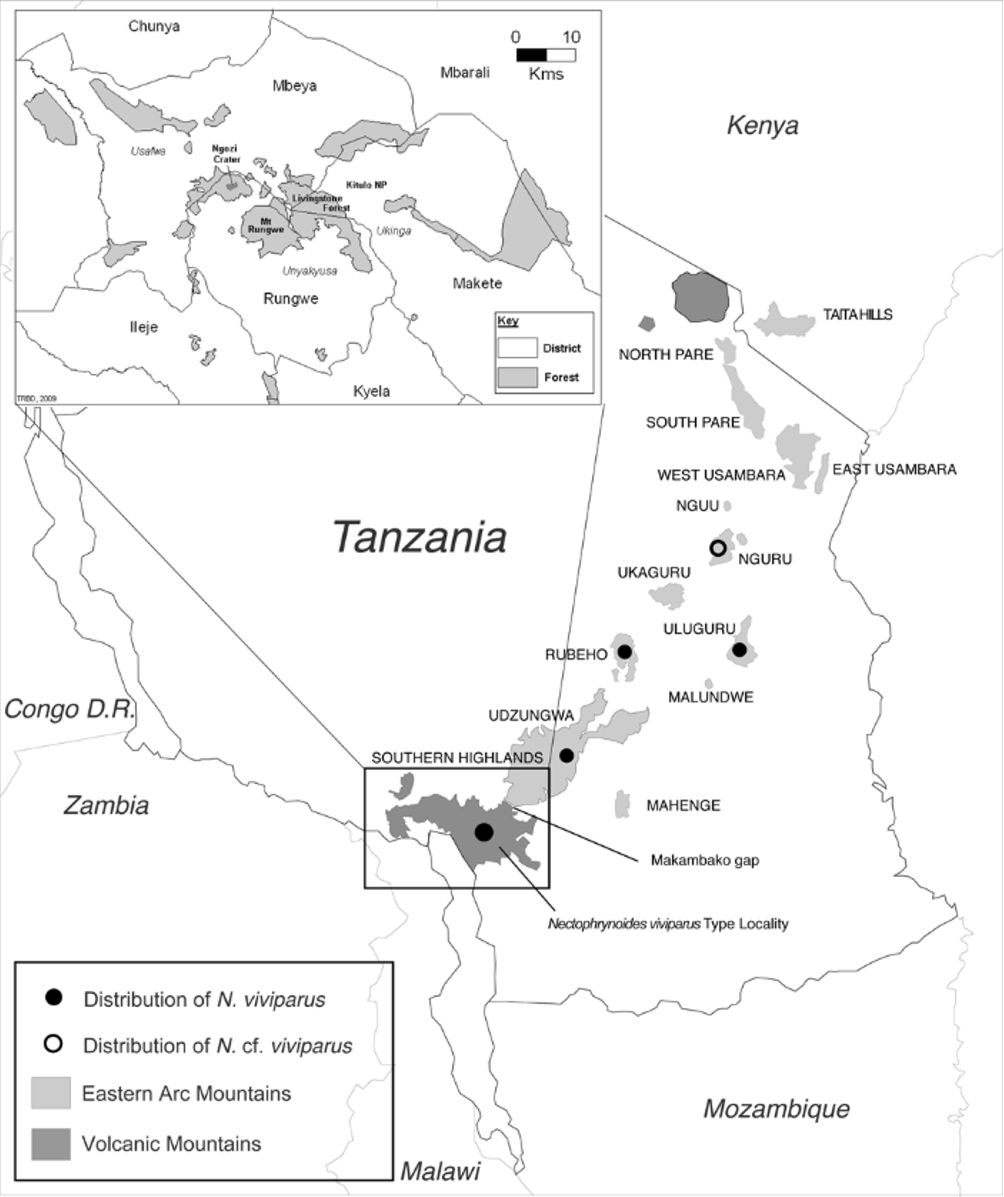
Type locality Kratersee des Nyisilvulkans (Ngosi Crater Lake, Fig. 1 View FIGURE 1 ).
Translation from original text: Species collected from various localities in German East Africa, i.e. Dar es Salam (collected by Werth and Emin Pascha), in Rungwe and the Kinga mountains (collected by S. Fülleborn). The specimens in which viviparity was detected originate from the collection donated to the museum by medical officer Dr. S. Fülleborn with the support of the Academy (of Sciences).
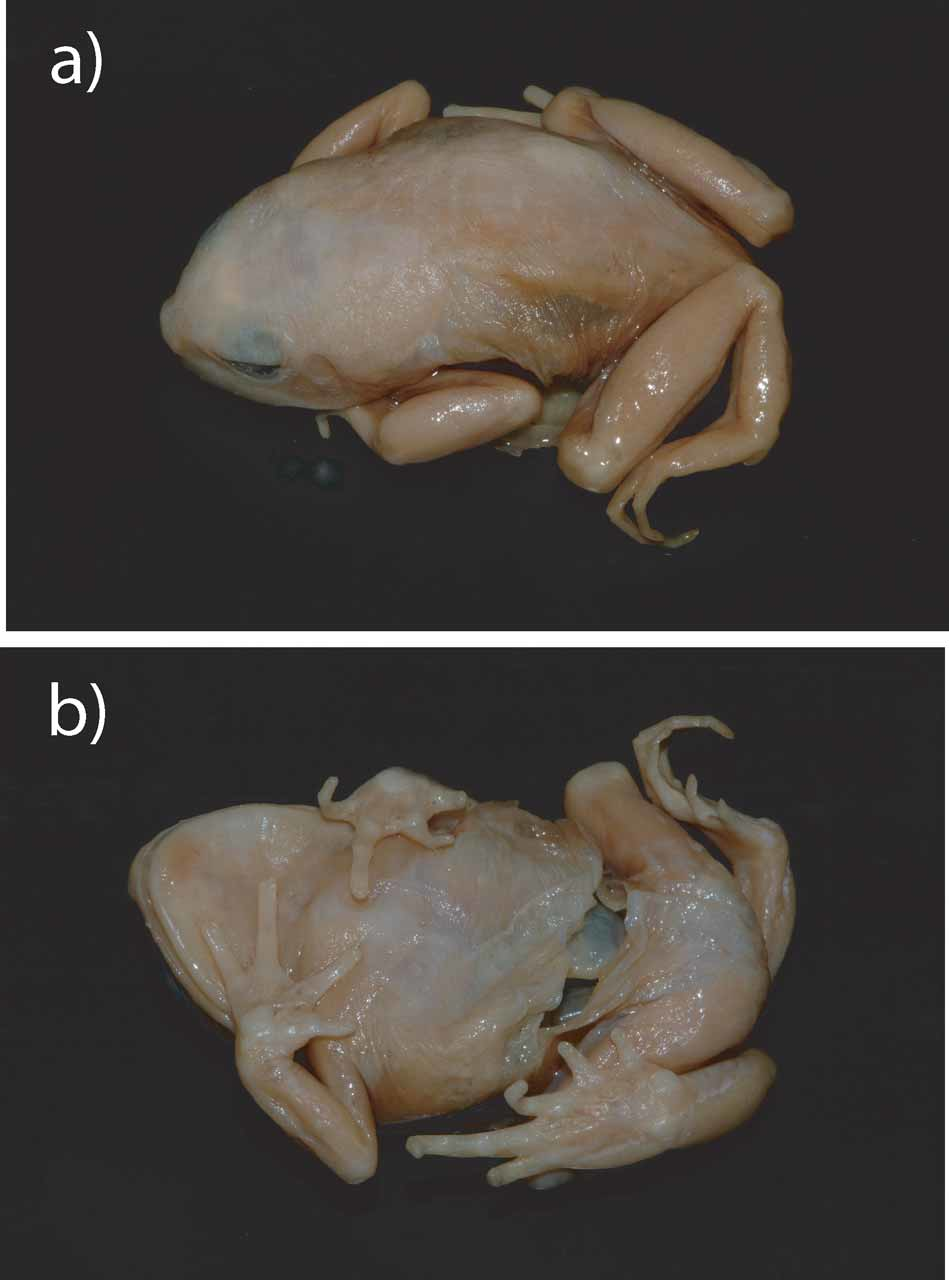
Description of the lectotype (measures in mm) ZMB 21775, adult female ( Fig. 2 View FIGURE 2 ). Dissected at lower mid ventral region. Embryos presumably removed and separately stored in small jar (18 embryos, 2.0-2.2 diameter= head and body including yolk; total length variable: 4.8-6.4). Distance from tip of snout to urostyle 32.9, width of head at jaw articulation 12.6, length of tibia 15.0, length of foot 14.4; tympanum and tympanic annulus easily discernible, ovoid shape 1.5 in diameter; parotoid glands present as a smooth glandular mass, 7.8 in length and 5.4 at widest point starting on the dorsal surface just behind the eye, directed posteriorly; snout relatively short, nostrils closer (2.2) to the snout tip than to each other (2.7) and also to the corner of the eyes (3.5); interorbital distance 6.7; eyes sunk and just about visible ventrally; eye diameter 3.2 right and left, width of eyelid, right 2.7 and left 2.3; interorbital distance 3.8 (difficult to measure as eyes are almost sunken into skull); canthus rostralis concave; arms and legs relatively slender; continuous glandular mass on both arms and legs; on arms, glands are continuous on dorsal, lateral and ventral surfaces of radius/ulna, not present on humerus; on legs, glands are continuous on dorsal, lateral and ventral surfaces of tibiofibulae and tarsal joints, not present on femur; continuous glandular mass on lateral and dorsal margin of the foot, attenuating to the margin of the phalange; rudimentary webbing on hands and feet; first finger shortest with second and fourth equal in size shorter than third finger which is the longest (6.4); first and second toe equal in size, third and fifth toes equal and larger, with fourth toe longest (10.5); small inner metatarsal tubercle, and large expanded outer metatarsal tubercle (1.8), shortest toe 3.0; the body skin is smooth both on glandular and non-glandular areas.
Colour pattern of lectotype in preservative Dorsal ground colour light brown with lighter cream patches on head region and parotoid glands; dark brown patches on posterior lateral margins, femoral and humerus regions of the specimen; glands present on lower arm, tibia and foot; ventral colour pattern is cream coloured, slightly white on the centre of ventral region.
Variation in material The material from Berlin comprises a range of embryos and adult specimens (SUL: 10.8-32.9). The addition of SHCP material includes adult specimens (SUL: 32.0-40.0). The larger size reported by Channing & Howell (2006) for N. viviparus is based on 166 specimens collected by A. Loveridge from the Ulugurus (e.g. males reach max SUL 56, and females c. 60; Barbour & Loveridge 1928; Channing & Howell 2006). It is unclear whether there are significant geographical differences in SUL between populations. This will require examination of specimens across all the assumed distribution range of the species (e.g. Ulugurus, Udzungwa and Rubeho; Channing & Howell 2006). We present morphometric data and SUL ratios for key measures for the specimens of N. viviparus from the Southern Highlands ( Table 2 View TABLE 2 ).
Barbour & Loveridge (1928) noted colour variation in Uluguru material of Nectophrynoides viviparus and hinted at a correlation with the habitats they were found – or “very slowly” changing their colouration to suit their habitat. Colour range from jet-black dorsal and ventral regions with glands marked rusty brown. Variations of this include dorsal regions coloured greenish, rufous-brown, bright yellow, in patches. The colour of the glands vary from rusty brown, to a lighter cream colour. In topotypic material colours from the original type series are all discoloured but Tornier (1905) provided some brief comments on their colour at the time of their descriptions (see Appendix 1) and remarked that juveniles are uniform blackish brown. Dorsal parts of back and legs fade as well to a colour that is clear chocolate brown. Dorsal surfaces are irregularly blackish brown and clear chocolate brown. From a recent collection in the Southern Highlands we noted colour variation ( Fig. 3 View FIGURE 3 ). For dorsal and ventral surfaces we observed specimens jet black with cream light brown coloured glands. The chin region was often also lighter coloured being a cream light brown. Differences to this pattern included specimens with a mid dorsal reddish-brown colouration and ventral region reddish brown. We also observed specimens with a light brown/yellow colouration completely covering dorsal and ventral aspects ( Fig. 3 View FIGURE 3 ).
Habitat information Nectophrynoides viviparus has been collected in montane forest from approx. 1800 to 2700 m a.s.l. This includes wet, open, secondary and disturbed montane forest, including logged Hagenia forest. In addition specimens have been collected in montane grassland and ericaceous heathland near the forest edge.
TABLE 2. Morphometric characters and ratios of Nectophrynoides viviparus (27 specimens from Tornier’s original series - excluding N. werthi specimens - see Table 1 - and five from topotypic material); measures in mm, and abbreviations Min. (Minimum), and Max. (Maximum).
| SUL | Average 22.6 | Min. 10.8 | Max. 40.0 | Number 32 |
|---|---|---|---|---|
| HL | 8.3 | 4.3 | 14.0 | 32 |
| HW ND | 8.4 2.4 | 4.1 1.2 | 15.0 4.5 | 32 32 |
| FL | 6.1 | 3.0 | 11.0 | 32 |
| TL ML | 9.7 6.3 | 4.5 3.0 | 18.0 12.0 | 32 32 |
| FOL | 9.5 | 4.0 | 19.0 | 32 |
| TYMP HL/SUL | 1.1 0.38 | 0.4 0.23 | 2.5 0.43 | 30 32 |
| HW/SUL | 0.37 | 0.32 | 0.42 | 32 |
| ND/SUL FL/SUL | 0.11 0.27 | 0.08 0.24 | 0.13 0.31 | 32 32 |
| TL/SUL | 0.42 | 0.38 | 0.50 | 32 |
| ML/SUL FOL/SUL | 0.28 0.41 | 0.23 0.31 | 0.31 0.53 | 32 32 |
| TYMP/SUL | 0.05 | 0.03 | 0.07 | 30 |
| SHCP |
Southern Highlands Conservation Programme |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Nectophrynoides viviparus ( Tornier, 1905 )
| Loader, Simon P., Poynton, John C., Davenport, Tim R. B. & Rödel, Mark-Oliver 2009 |
Nectophrynoides viviparus
| Anon. 1996 |
Nectophrynoides viviparus
| Perret 1971 |
vivipara
| Miranda-Ribeiro 1926 |
Nectophrynoides werthi
| Nieden 1910 |
Pseudophryne vivipara
| Tornier 1905 |