Elphidium de Monfort 1808
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4215.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:B91D1782-C11A-4CDC-96B6-76104FEE51BD |
|
DOI |
https://doi.org/10.5281/zenodo.6067899 |
|
persistent identifier |
https://treatment.plazi.org/id/0389064B-FF9D-3D02-3EEE-E3DDFAFDB8B0 |
|
treatment provided by |
Plazi |
|
scientific name |
Elphidium de Monfort 1808 |
| status |
|
Elphidium de Monfort 1808 View in CoL
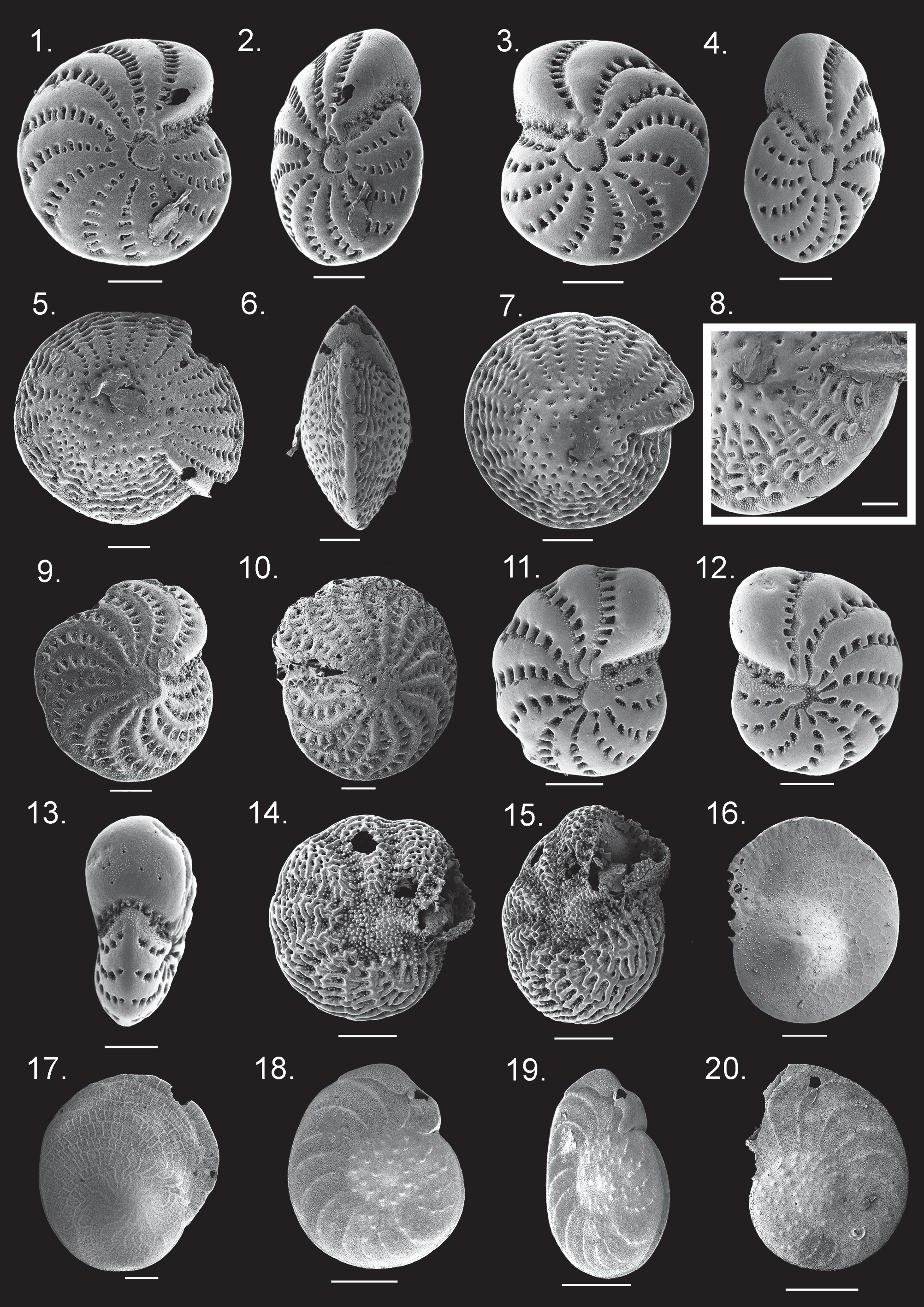
Elphidium advenum ( Cushman 1922) View in CoL ( Fig. 25 View FIGURE 25 :1–4)
1884 Polystomella subnodosa (Münster) ; Brady, p. 734, pl. 110, fig. 1.
1922 Polystomella advena Cushman , p. 56, pl. 9, figs 11, 12.
1933 Elphidium advenum (Cushman) , p. 50, pl. 12, figs 1–3.
1939 Elphidium advenum (Cushman) ; Cushman, p. 60, pl. 16, figs 31–35.
1992a Elphidium advenum (Cushman) ; Hatta & Ujiié, p. 203, pl. 49, figs 3, 4. 1993 Elphidium cf. E. advenum (Cushman) ; Hottinger et al., p. 146, pl. 207, figs 1–7. 1994 Elphidium advenum (Cushman) ; Loeblich & Tappan, p. 168, pl. 379, figs 1–4. 1995 Elphidium advenum (Cushman) ; Lobegeier, p. 84, pl. 6, figs 10, 11.
1995 Elphidium advenum (Cushman) ; Yassini & Jones, p. 176, figs 1026–1029, 1034–1036. 1997 Elphidium advenum limbatum (Chapman) ; Hayward et al., p. 67, pl. 3, figs 11–16. 1999 Elphidium advenum f. limbatum (Chapman), Hayward et al. p. 164, pl. 17, figs 1, 2. 2002 Elphidium advenum (Cushman) ; Bicchi et al., p. 282, fig. 7: 16.
2010 Elphidium advenum (Cushman) ; Narayan & Pandolfi, p. 2076, pl. 1, fig. 15, 16. 2012 Elphidium advenum (Cushman) ; Debenay, p. 218, pl. 19.
Description. See Cushman (1922, p. 56, pl. 9, figs 11, 12) and Hayward (1997, p. 67, pl. 3, figs 11–16; 1999, p. 164, pl. 17, figs 1, 2).
Remarks. This widespread taxon has an equally biconvex, lenticular test with a rounded outline and acute peripheral margin. The test normally has 11–14 chambers that are slightly inflated and the final chamber tends to be lobulate in adult specimens. Retral processes span depressed sutures, which are radial and curve slightly backwards. The test wall is smooth with minute papillae clustering around the sutures and retral processes at the base of the umbilical face and central boss. In amongst the papillae, the apertures are a series of pores along the base of the umbilical face ( Fig. 25 View FIGURE 25 :1¯4).
Elphidium advenum ( Cushman 1922) View in CoL can be distinguished from other Elphidium View in CoL species by its smooth test with comparatively fewer chambers and rounded, slightly keeled periphery. Elphidium advenum View in CoL most closely resembles Elphidium maorium Hayward 1997 View in CoL in regards to test shape, number of chambers, retral processes and papillate ornament. However, E. maorium View in CoL has more inflated chambers and no peripheral carina. The final chamber of E. maorium View in CoL is distinctly broader and more inflated than the final chamber of E. advenum View in CoL and also has large perforations high up on the umbilical face with clustered papillae.
Intraspecific variation in E. advenum View in CoL includes the thickness and size of the carina. In some cases the carina is slender, giving the test periphery an almost knife-like edge ( Cushman 1922). In other forms, it is greatly produced and is almost keel-like ( Loeblich & Tappan 1994; Yassini & Jones 1995; Debenay 2012) or it is subdued and only evident in profile view. The CG specimens have both thick ( Hatta & Ujiié 1992a) ( Fig. 25 View FIGURE 25 :1, 2) and slender ( Hottinger et al. 1993; Lobegeier 1995; Hayward et al. 1997; Hayward et al. 1999) ( Fig. 25 View FIGURE 25 :3, 4) carina.
Hayward et al. (1997; 1999) published Elphidium advenum f. limbatum ( Chapman 1907) and synonymised Polystomella macellum var. limbatum Chapman 1907 , as the holotype and first publication of this form of the species. Polystomella macellum var. limbatum , whilst bearing similar shape and ornament to E. advenum , has prominent, inflated chamber walls, as opposed to depressed sutures which raise above the retral processes. Specimen illustrations by Hayward et al. (1997, pl. 3, figs 9–17, pl. 4, figs 1–10; 1999, pl. 17, figs 3–5) clearly possess both of these morphotypes. The type locality established by Cushman for E. advenum is not designated beyond the region of the Tortugas ( Cushman 1922; Ellis & Messina 1940), but localities given by Cushman (1922) include Loggerhead Key , East Key and Texas Rock, Dry Tortugas Islands , Florida from depths ranging from 10 – 21 m. Hatta & Ujiié (1992a) collected E. advenum from between Ishigaki and Iriomote Islands , Ryukyu Island Arc and Hottinger et al. (1993) from the Gulf of Aqaba , Red Sea. Loeblich & Tappan (1994) reported this species from one sample at a depth of 71 m, north of Cape Londonderry, southern Timor Sea and Lobegeier (1995) from Low Isles Reef, GBR. Yassini & Jones (1995) collected E. advenum from inlet channels, open estuarine, sheltered oceanic embayments, intertidal zone and the inner shelf with rare occurrences in coastal lagoons from southeastern Australia . Bicchi et al. (2002) recovered the taxon from central lagoon settings in the central Tuamoto Archipelago , French Polynesia . Hayward et al. (1997; 1999) found E. advenum widespread throughout New Zealand including Kermadec, Chatham, Auckland and Campbell Islands as a slightly brackish, low tidal or shallow subtidal species within enclosed harbours and tidal inlets. The taxon also occurrs in fully marine but moderately sheltered inner shelf locations and is rare in exposed, deeper water sites in and around New Zealand (1997; Hayward et al. 1999) and in river delta sands of Moreton Bay , Southern GBR ( Narayan & Pandolfi 2010). Debenay (2012) found this species from the southwestern lagoon of New Caledonia at depth 30 m.
Distribution within study area. Elphidium advenum is the most abundant Elphidium species collected from the CG. Interestingly, it is absent from the channel samples and Sykes Reef and occurs in comparatively low numbers in the shallow reef flat samples from Heron Reef flat. Wistari Lagoon had the highest average number of specimens (twenty-six per site) and the highest abundance occurred at site 21. Live specimens of E. advenum were also collected, but there was never more than one live specimen per site which included four sites from Heron Reef flat, One Tree Lagoon 2 and Wistari Lagoon.
Elphidium craticulatum ( Fichtel & Moll 1798) View in CoL ( Fig. 25 View FIGURE 25 :5–8)
1798 Nautilus craticulatum Fichtel & Moll , p. 51, pl. 5, figs h–k.
1992a Cellanthus craticulatus (Fichtel & Moll) ; Hatta & Ujiié, p. 203, pl. 49, fig. 7.
1993 Elphidium craticulatum (Fichtel & Moll) ; Hottinger et al., p. 147, pl. 208, figs 1–10; pl. 209, figs 1–6; pl. 210, figs 1–6. 1993 Elphidium craticulatum (Fichtel & Moll) ; Mather & Bennett, p. 32, fig. 11: 15.
1994 Cellanthus craticulatus (Fichtel & Moll) ; Loeblich & Tappan; p. 167, pl. 380, figs 1–10. 1995 Elphidium craticulatum (Fichtel & Moll) ; Lobegeier, p. 84, pl. 6, figs 12–14.
1997 Elphidium craticulatum (Fichtel & Moll) ; Hayward et al., p. 72, pl. 7, figs 5–12. 2009 Elphidium craticulatum (Fichtel & Moll) ; Parker, p. 575, fig. 405a–e.
2010 Elphidium craticulatum (Fichtel & Moll) ; Narayan & Pandolfi, p. 2076, pl. 1, fig. 17. 2012 Elphidium craticulatum (Fichtel & Moll) ; Debenay, p. 219, pl. 19.
Description. See Hayward et al. (1997, p. 72, pl. 7, figs 5–12), Hottinger et al. (1993, p. 147, pl. 208, figs 1–10: pl. 209, figs 1–6; pl. 210, figs 1–6) and Parker (2009, p. 575, fig. 405a–e).
Remarks. Elphidium craticulatum ( Fichtel & Moll 1798) is characterised by a large, equally biconvex, thick lenticular test with an acute periphery and a large, perforate umbilical plug. Depressed sutures are straight and covered by numerous retral processes. Chambers are numerous (between twenty and forty) and inflated with apertures and retral processes covered by numerous papillae. The base of the apertural face is also lined with fine beads ( Fig. 25 View FIGURE 25 :8), with shallow anastomising striae extending to the test periphery. The central plug or boss is coarsely perforate and approximately a third to half the diameter of the test ( Fig. 25 View FIGURE 25 :5–7).
Parker (2009) highlighted the taxonomic confusion between this taxon and Cellanthus de Montfort 1808 ( Hatta & Ujiié 1992a; Loeblich & Tappan 1994). This stems from Loeblich & Tappan (1987) who indicated Cellanthus should be regarded as a junior synonym of Elphidium because the genus possesses a perforate, as opposed to an imperforate, umbilical plug. Detailed morphological investigations by Hottinger et al. (1993) shed no further light on this question and so, for the time being, a conservative approach is taken here and the species is retained in Elphidium .
Elphidium craticulatum View in CoL differs from other Elphidium View in CoL species by its large size, greatly inflated test, numerous chambers, straight depressed sutures and very large umbilical boss. However, the size of the umbilical boss in specimens of E. craticulum from the CG display considerable intraspecific variation and ranges in width from a third to half the diameter of the test ( Fig. 25 View FIGURE 25 :5, 7). Other published examples of E. craticulum display a similar variation in boss size relative to the test ( Hatta & Ujiié 1992a; Hottinger et al. 1993; Lobegeier 1995; Hayward et al. 1997; Parker 2009). However, specimens published by Loeblich & Tappan (1994) and Narayan & Pandolfi (2010) illustrate bosses that range up to almost two-thirds test diameter.
The type locality for E. craticulatum View in CoL is the Arabian Sea and Indian Ocean ( Fichtel & Moll 1798) and has since been described from a global distribution (Ryukyu Island Arc—Hatta & Ujiié 1992a; Gulf of Aqaba, Red Sea— Hottinger et al. 1993; Timor Sea from 31–131 m—Loeblich & Tappan 1994; GBR—Mather & Bennett 1993, Lobegeier 1995, Pandolfi 2010; New Caledonia to 30 m—Debenay 2012; Ningaloo Reef—Parker 2009). Hayward et al. (1997) described E. craticulatum View in CoL as a common low tidal to shallow subtidal (0–20 m) species living in clean sands with normal marine salinities around the northeast coast of Australia and tropical islands of the south-west Pacific and with strong faunal associations with species that include, amongst others, Textularia View in CoL , Reophax , Quinqueloculina View in CoL , Spiroloculina View in CoL , Triloculina View in CoL , Peneroplis View in CoL , Amphistegina View in CoL , Baculogypsina View in CoL , Reusella , Bolivina View in CoL , Discorbis View in CoL , Operculina View in CoL and other Elphidium View in CoL .
Distribution within study area. Elphidium craticulatum was the third most abundant Elphidium from the CG and was collected from all sampled reefs. Abundance ranged from zero to twenty-three specimens per site with highest abundance at site 12 in Wistari Lagoon. However, the average number of specimens per site was generally lower on Heron Reef flat than in lagoon localities.
Elphidium macellum ( Fichtel & Moll 1798) View in CoL ( Fig. 25 View FIGURE 25 :9, 10)
1798 Nautilus macellus var. α Fichtel & Moll, p. 66, pl. 10, figs e–g.
1984 Elphidium macellum (Fichtel & Moll) , Rögl & Hansen, p. 39, pl. 9, figs 3–4, textfig. 12. 1995 Elphidium macellum (Fichtel & Moll) ; Yassini & Jones, p. 177, figs 1020, 1022–1025. 1997 Elphidium macellum (Fichtel & Moll) ; Hayward et al., p. 84, pl. 13, figs 9–14. 2009 Elphidium cf. E. macellum (Fichtel & Moll) ; Parker, p. 582, figs 410a–e.
2012 Elphidium macellum (Fichtel & Moll) ; Debenay, p. 220, pl. 19.
Description. See Rögl & Hansen (1984 p. 39, pl. 9, figs 3, 4, textfig. 12) and Hayward et al. (1997, p. 84, pl. 13, figs 9–14).
Remarks. Elphidium macellum ( Fichtel & Moll 1798) is characterised by an involute, equally biconvex test with a uniformly acute periphery that hosts a rounded keel. Chambers are numerous (fifteen to twenty-two) and the anterior area of each chamber possesses a radiating rib that curves backwards to the peripheral keel that traces the chamber outline. The sutures are depressed and covered by numerous septal bridges. Small, faint papillae cluster within sutures and along the edges of the keel. The umbilical area is flat and unornamented ( Fig. 25 View FIGURE 25 :9, 10). Elphidium macellum from the CG differs from other published examples by having a flat, rather than depressed, umbilical area (e.g. Parker 2009). In all other respects, E. macellum from the CG conforms to the description for the taxon provided by Rögl & Hansen (1984) and Hayward (1997).
Elphidium macellum can be distinguished from other Elphidium species based on the almost equal proportion of chamber width to the suture depression, as opposed to the sutures being significantly thinner than the chamber width in other taxa. The numerous chambers and radiating ribs that trace each chamber to the keel also make this species distinct from similar species. In addition, the periphery of the umbilical face is lined with a shallow, wide, raised circular ornament that is not found on any other Elphidium species ( Fig. 25 View FIGURE 25 :9, 10).
The type locatity for E. macellum is the Mediterranean Sea ( Ellis & Messina 1940) and has a global distribution ( Gross 2004; South Australia—Yassini & Jones 1995; Ningaloo Reef , Western Australia—Parker 2009; New Caledonia to 30 m—Debenay 2012). Yassini & Jones (1995) described this taxon from open estuaries, sheltered oceanic embayments, intertidal zone of the inner shelf and on rare occasions from inlet channels of coastal lagoons along the southeastern coast of Australia. Hayward et al. (1997) described E. macellum as a common species in moderately sheltered beach and inner shelf depths throughout the southwest Pacific. On occasion, this taxon has been collected from bathyal depths as reworked elements, but is more frequently a major component of low tidal and shallow subtidal assemblages associated with other genera including Textularia , Quinqueloculina , Spiroloculina , Triloculina , Discorbis and other Elphidium species. This taxon is also common in tropical environments especially within coral-enclosed lagoons, bays and outer reef slopes where it occurs with Amphistegina , Baculogypsina , Tinoporus , Discorbis , Operculina , Peneroplis , miliolines and other Elphidium ( Hayward et al. 1997) .
Distribution within study area. Elphidium macellum is the fourth most abundant Elphidium species collected and its abundance per site is normally greater than seven specimens. This species was absent from Sykes Reef, but is otherwise spread relatively evenly throughout the CG. The site of greatest abundance was at site 10 along ST/ HW transect at the rampart/reef flat edge of Heron Reef. One live specimen of E. macellum was collected from site 12 in Wistari Lagoon.
Elphidium maorium Hayward 1997 View in CoL ( Fig. 25 View FIGURE 25 :11–13)
1997 Elphidium advenum maorium Hayward View in CoL , p. 69, pl. 1, fig. 7; pl. 4, figs 11–16, pl. 5 figs 1–5. 2012 Elphidium maorium Hayward, Debenay View in CoL , p. 220, pl. 19.
Description. See Hayward et al. (1997, p. 69, pl. 1, fig. 7; pl. 4, figs 11–16, pl. 5 figs 1–5) and Debenay (2012, p. 220, pl. 19).
Remarks. Elphidium maorium Hayward 1997 has a circular to lobulate test with a compressed profile and broadly rounded periphery. There are 9–10 inflated chambers with depressed sutures that gently curve in a backwards direction and possess septal bridges. The septal bridge sides and suture walls are covered by fine, scattered papillae. The test wall is very finely perforate. The umbilical face is clean and bears apertural tubercules in the umbilical depression. Some large, supplementary pores are scattered across the central third of the umbilical face with faint papillate ornament. The umbilical boss is low, small and surrounded by a depressed canal filled with papillae which connect to the sutures ( Fig. 25 View FIGURE 25 :11–13).
Specimens collected from the CG vary slightly from those illustrated by both Hayward et al. (1997) and Debenay (2012) as CG specimens lack an imperforate band around the periphery that gives the test a more acutely rounded edge and a peak to the apertural face in profile.
Elphidium maorium can be distinguished from other Elphidium species by its low number of chambers and lobulate outline ( Fig. 25 View FIGURE 25 :11–13). This species most closely resembles E. advenum , but E. advenum has a more compressed test, acute periphery with a carina, possesses more chambers and the umbilical face is not as high, nor are there any large pores in the central third of the umbilical face and is more triangular in shape than rounded.
The extent of the papillate ornament in E. maorium varies quite substantially such that morphologically identical specimens collected from the CG can have substantially more papillae ( Fig. 25 View FIGURE 25 :11–13) than those described elsewhere which have fewer papillae or different distribution in comparison ( Hayward et al. 1997; Debenay 2012).
The type locality for E. maorium is Parnell Reef, Auckland, New Zealand and is distributed throughout the Southwest Pacific ( New Zealand, Chatham Islands, eastern Australia, Kermadec, Norfolk and Lord Howe Islands—Hayward 1997; New Caledonia at 10 m—Debenay 2012). Its ecological distribution in New Zealand is rare in sheltered nearshore, normal marine salinity locations but sometimes common in slightly brackish ares in harbors and inlets ( Hayward et al. 1997). Around Southwest Pacific locations and in the CG, they are found in shallow/inter-tidal depths in normal marine salinity ( Hayward et al. 1997; Debenay 2012).
Distribution within study area. Elphidium maorium is the second most abundant Elphidium species collected from the CG and was almost exclusively restricted to lagoon environments. Abundance is greatest around a depth window of 2.5– 5 m. The site of greatest abundance is site 34 in One Tree Lagoon 1. Several E. maorium specimens were collected live from One Tree and Wistari Lagoons with up to three live specimens found at site 47 in One Tree Lagoon 3.
Elphidium milletti ( Heron-Allen & Earland 1915) View in CoL ( Fig. 25 View FIGURE 25 :14, 15)
1904 Polystomella verriculata Brady ; Millett, p. 604, pl. 11, fig. 3.
1915 Polystomella milletti Heron-Allen & Earland , p. 735, pl. 53, figs 38–42.
1993 Parrellina ? cf. P.? milletti (Heron-Allen & Earland) ; Hottinger et al., p. 152, pl. 218, figs 1–9; pl. 219, figs 1–4. 2009 Elphidium milletti (Heron-Allen & Earland) ; Parker, p. 582, figs 411a–i, 412a–f. 2012 Elphidium milletti (Heron-Allen & Earland) ; Debenay, p. 221, pl. 19.
Description. See Heron-Allen & Earland (1915, p. 735, figs 38–42), Hottinger et al. (1993, p. 152, pl. 128, figs 109; pl. 219, figs 1–4) and Parker (2009, p. 582, figs 411a–i, 412a–f).
Remarks. Elphidium milletti ( Heron-Allen & Earland 1915) has a planispiral test with a lobate, rounded periphery and 10–15 inflated chambers. Sutures and the umbilical area are depressed, but this species has a distinctive ornament consisting of numerous, large papillae covering the depressed sutures so that the sutures and the retral processes that cross them are obscured. The radiating sutures curve back towards the periphery and the umbilical area is also covered by papillae. The chamber walls are finely perforate, but are also obscured by thick, strong, raised, ridged ornaments that traverse the chamber. Ridges can become chevron-shaped when connected, particularly in the final larger chambers. The umbilical face, though difficult to discern due to the damaged state of most tests, is covered by small, short, produced tubercules that also line the depressed umbilicus at the base ( Fig. 25 View FIGURE 25 :14, 15). The striking ornament of E. milletti serves to distinguish this species from other Elphidium species.
Elphidium milletti View in CoL is not a widely recorded species, but morphological differences between specimens from the CG and other localities occurs in the bead and ridge ornamentation ( Parker 2009). Some specimens possess more prolific ‘beads’ ( Heron-Allen & Earland 1915) and the depressed sutures appear much wider ( Parker 2009) or, as in the CG and Debenay’s (2012) specimens, the “beads” only occur within narrow channels and are partially obscured by septal bridges and the raised ornament ( Fig. 25 View FIGURE 25 :14, 15). The raised ridges can also be weakly expressed or more widely spaced making the ‘chevron’ sutures difficult to discern ( Hottinger et al. 1993; Parker 2009). The sutures in CG specimens are closely spaced and well developed which allows the ‘chevron’ pattern to be easily discerned ( Fig. 25 View FIGURE 25 :14) ( Heron-Allen & Earland 1915; Debenay 2012).
The type location for E. milletti View in CoL is not clearly stated, but the general area seems to be the Kerimba Archipelago, Mozambique ( Ellis & Messina 1940). Hottinger et al. (1993) reported a small number of specimens from the Gulf of Aqaba , Red Sea, and Parker (2009) described E. milletti View in CoL from Ningaloo Reef , Western Australia . Parker (2009) also noted that this taxon is known from the Philippines, GBR and southwest Japan. Debenay (2012) collected E. milletti View in CoL from the southwestern lagoon of New Caledonia from a depth of 40 m.
Distribution within study area. Elphidium milletti was collected exclusively from Heron and One Tree Lagoons. Abundance was usually one to five specimens per site, but the greatest abundance (19 specimens) was found at site 34 located in the northwestern extremity of One Tree Lagoon 1 in a labyrinth of patch reefs.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Elphidium de Monfort 1808
| Mamo, Briony L. 2016 |
Elphidium maorium
| Hayward 1997 |
Elphidium maorium
| Hayward 1997 |
Elphidium advenum (
| Cushman 1922 |
Elphidium advenum (
| Cushman 1922 |
Elphidium milletti (
| Heron-Allen & Earland 1915 |
Elphidium craticulatum (
| Fichtel & Moll 1798 |
Elphidium macellum (
| Fichtel & Moll 1798 |