Contarinia ampelitsiae Dorchin & Fazan, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5301.2.6 |
|
publication LSID |
lsid:zoobank.org:pub:E47DA010-607C-402D-953E-3D9F3E2FD460 |
|
DOI |
https://doi.org/10.5281/zenodo.8030552 |
|
persistent identifier |
https://treatment.plazi.org/id/0389878F-3479-700E-7897-FF06FA81BD84 |
|
treatment provided by |
Plazi |
|
scientific name |
Contarinia ampelitsiae Dorchin & Fazan |
| status |
sp. nov. |
Contarinia ampelitsiae Dorchin & Fazan , new species
Diagnosis. This species is placed in Contarinia for the binodal male flagellomeres, each with a single whorl of circumfila, untoothed tarsal claws, long, retractable and tapered ovipositor with greatly reduced, closely juxtaposed cerci, and 4 pairs of larval terminal papillae, two of which are coniform and recurved. The species has no obvious relatives within Contarinia , given that it forms previously unknown, elaborate stamen galls on Ulmaceae , a plant family from which no Contarinia species are known. No species of Contarinia are known also from the closely related plant family Cannabaceae , formerly included in the Ulmaceae , from which numerous cecidomyiid species of other genera are known ( Gagné et al. 2013). Moreover, Contarinia ampelitsiae is univoltine, which excludes the possibility that it develops on other host plants. Adults have no dorsal occipital protuberance, the female antennal flagellomeres have very short necks, male antennal flagellomeres with necks as long as nodes, and larva with longshafted, bilobed spatula.
Description.
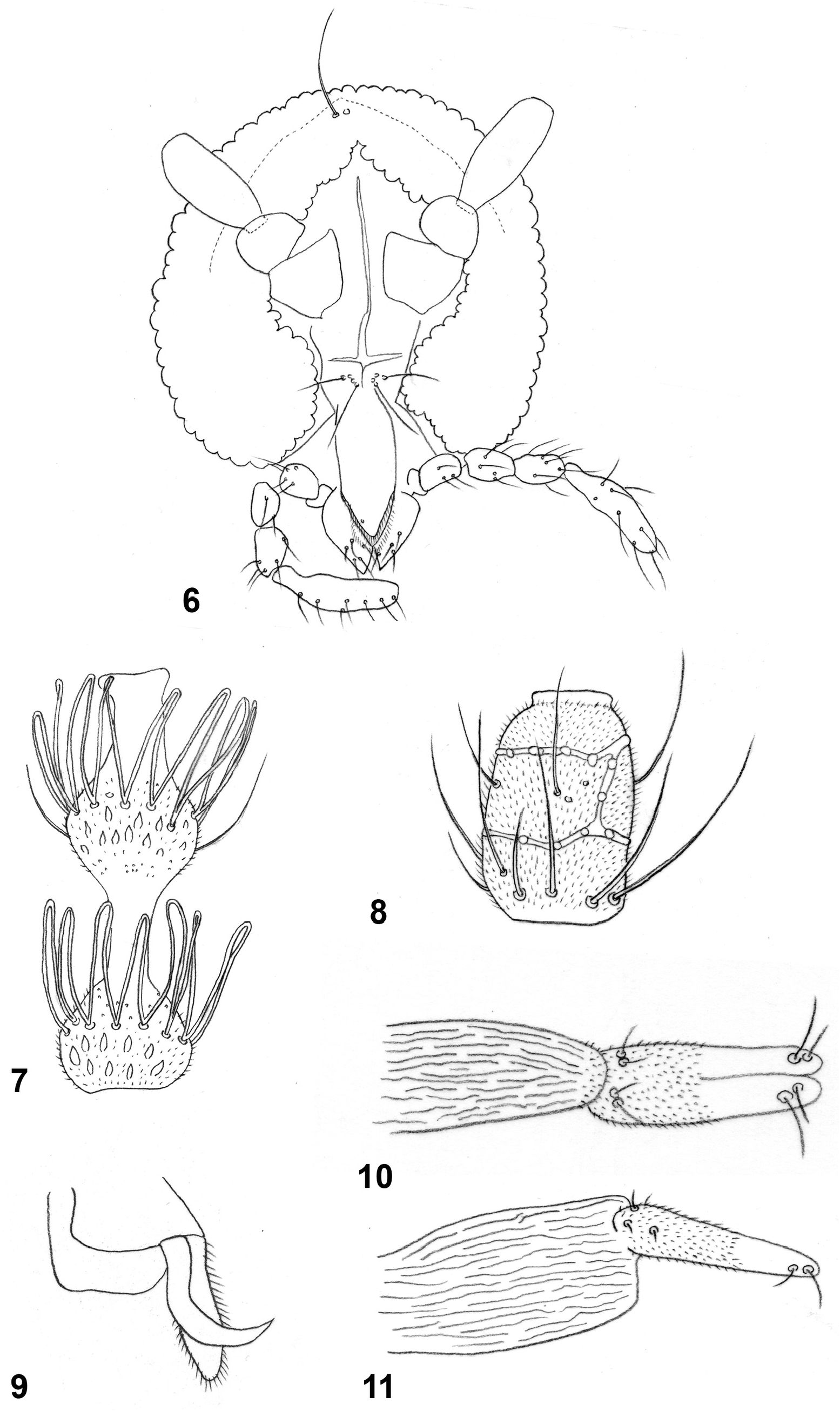
Adult. Head ( Fig. 6 View FIGURES 6–11 ): Eye facets round, more spacious laterally than medially. Occiput without dorsal protuberance; only slightly raised towards shallow, blunt projection carrying two long apical setae ( Fig. 6 View FIGURES 6–11 ). Frons with 4–5 long setae on each side. Labellum tapered from wide base, with several subapical setae. Palp four segmented, segments successively longer, palpiger present ( Fig. 6 View FIGURES 6–11 ). Male antennal flagellomeres ( Fig. 7 View FIGURES 6–11 ) with necks about same length as nodes, slightly longer in distal than in proximal flagellomeres; circumfilar loops reaching base of next node or slightly longer. Female flagellomeres ( Fig. 8 View FIGURES 6–11 ) with very short necks, each with two whorls of appressed circumfila connected by one longitudinal band.
Thorax: Wing transparent, with long, fine, hair-like setae along posterior margin, R 4+5 slightly bent, reaching C beyond wing apex. C with small break immediately past juncture with R 4+5. Wing length 2.15–2.56 mm in females (n=3), 1.93–2.34 mm in males (n=4). Legs covered by long, narrow scales; tarsal claws ( Fig. 9 View FIGURES 6–11 ) untoothed, curved along distal half, empodia longer than bend in claw, pulvilli minute.
Female abdomen: Tergites 1–7 with anterior pair of sensory setae, posterior row of setae, and a group of 5–6 baso-lateral setae.Tergite 8 smaller than preceding, with few posterior setae. Sternites 2–7 with anterior pair of closely approximated sensory setae, continuous posterior row of setae and numerous evenly distributed setae laterally; pigmentation of sternites broken along thin line just proximal to posterior row of setae; sternite 8 undifferentiated from surrounding tissue. Ovipositor 10.7–12.3 as long as tergite 7 (n=3). Cerci ( Figs. 10–11 View FIGURES 6–11 ) closely appressed, about 4 times as long as wide, each bearing two strong setae apically and two smaller setae basally, microtrichose along proximal half, bare thereafter.
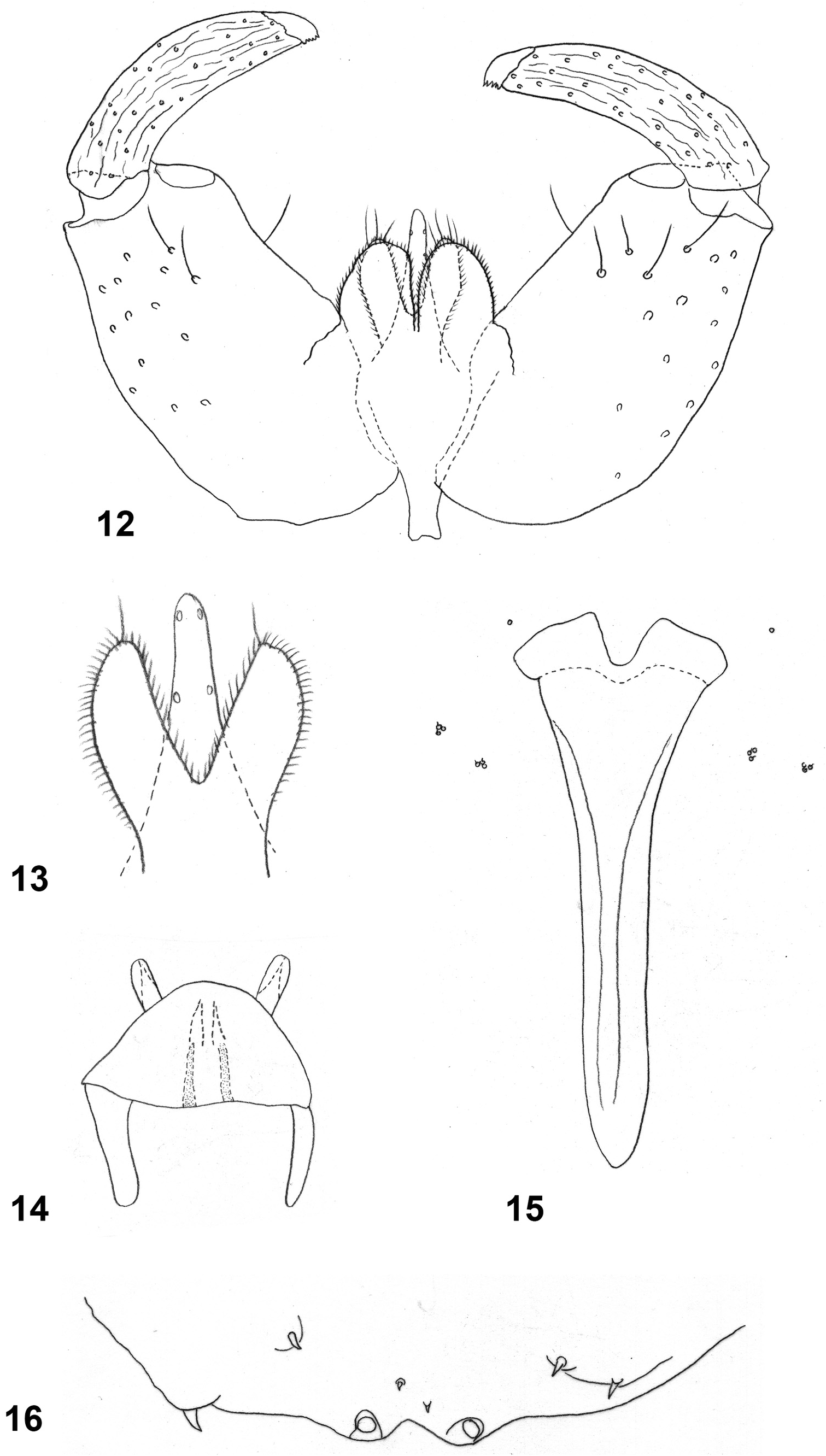
Male abdomen: Tergites 1–6 as in female; tergite 7 with only 2–3 lateral setae and without posterior row of setae; tergite 8 with much smaller sclerotized area, anterior pair of sensory setae the only vestiture. Sternites 2–6 as in female; sternites 7–8 without clear separation between lateral and posterior setae. Terminalia ( Figs. 12– 13 View FIGURES 12–16 ): Gonocoxites robust, cylindrical, with evenly distributed long setae; gonostyli about same width throughout length, only slightly narrowed towards apical tooth, entirely glabrous and carinate, with evenly distributed setae; cerci separate to base, with several apical setae; hypoproct deeply divided into two cylindrical lobes, strongly microtrichose, each with one long seta apically ( Fig. 13 View FIGURES 12–16 ); aedeagus longer than hypoproct, narrow, tapered to rounded apex, with two pairs of asetose sensory organs on each side.
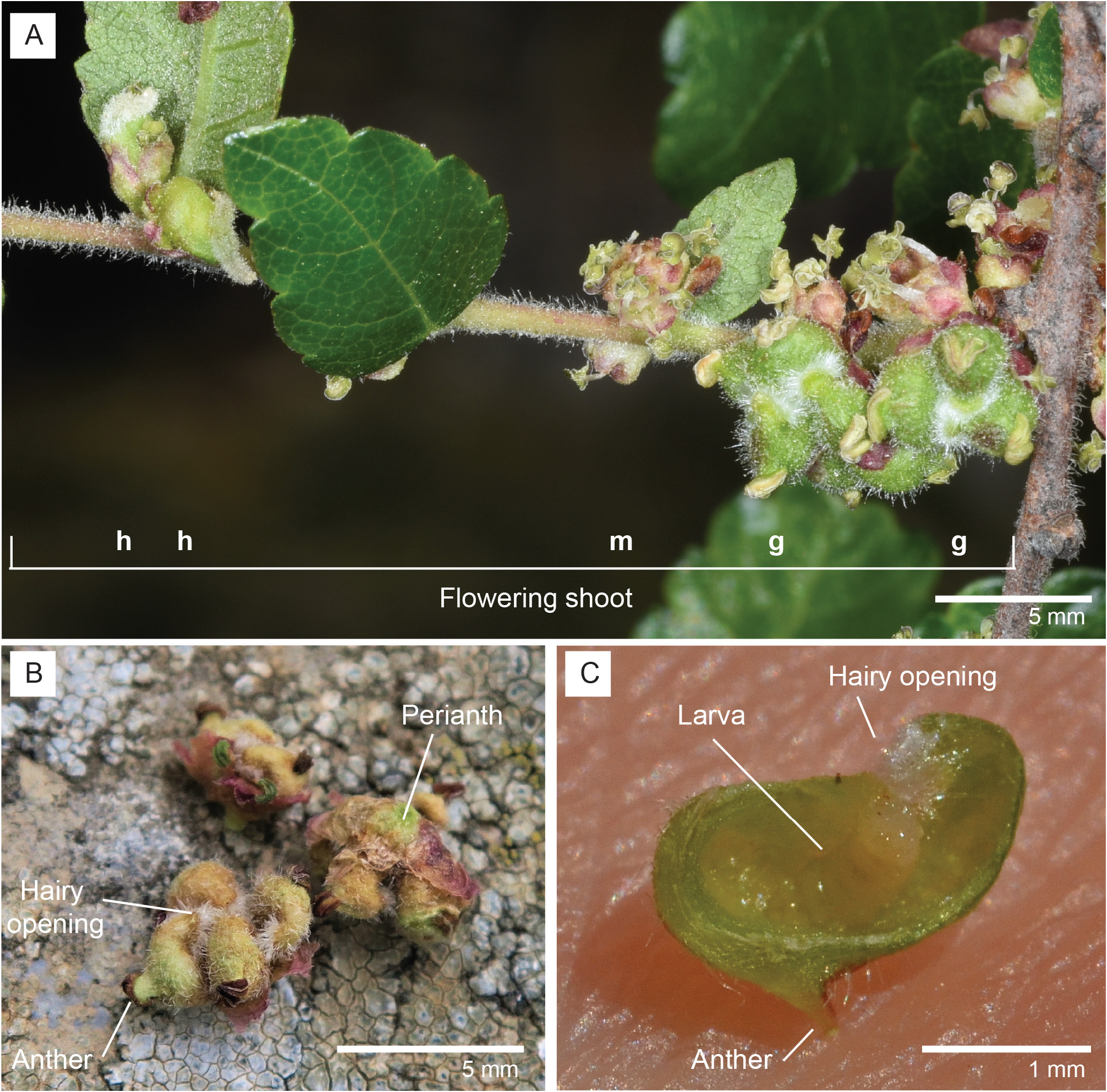
Larva (third instar) ( Fig. 3C View FIGURE 3 ): Length 3.17–3.81 mm (n=10), whitish to pale yellow; antenna about 1.5 times as long as wide, cephalic apodemes as long as head capsule ( Fig. 14 View FIGURES 12–16 ). Spatula ( Fig. 15 View FIGURES 12–16 ) long shafted, with two wide, laterally rounded, mesally slightly pointed lobes separated by deep depression; broadest at base of lobes. On each side of spatula two triplets of tiny papillae, two in each triplet with barely visible setae. Third thoracic segment and first to eighth abdominal segments each with ventral field of minute spicules arranged in 6–15 transverse rows on mid-anterior section of segment; integument otherwise smooth. Coniform terminal papillae recurved, other three pairs with short, thick setae on slightly elevated bases ( Fig. 16 View FIGURES 12–16 ).
Pupa (based on exuviae): A few degraded exuviae enabled only partial description. Antennal bases form very small and short tapered tips. Cephalic seta long and thin.
Etymology. The specific name refers to the local Cretan name for Z. abelicea , namely ampelitsiá (αμπελιτσιά).
DNA: The barcoding section of the mitochondrial COI gene sequenced from one male of C. ampelitsiae was deposited in GenBank under accession number OQ107473. Comparison of this sequence to available sequences in GenBank showed highest percentage identity of 91.03–91.73% with numerous unidentified cecidomyiid specimens, with the highest similarity to other Contarinia species being 91.18% to Contarinia caryafloralis from Carya in China (accession MH039840.1, Jiao et al. 2018), and 90.66% to an unidentified Contarinia species from Oxalis in Japan (accession AB597022.1, Uechi et al. 2011). These values indicate that Contarinia species for which relevant sequences are currently available in GenBank are very different from C. ampelitsiae and are unrelated to it.
Material examined: Holotype: ♀, Greece, Crete, Katharo plateau, 35.144263 N, 25.573718 E, 28.v.2020, I. Remoundou. Ex stamen galls of Zelkova abelicea . Emerged on 31.iii.2021. On permanent microscope slide in Euparal. Deposited in NHMF (accession GBIFCH01352024 ) GoogleMaps . Paratypes: All from Greece, Crete, Katharo plateau, 35.144263 N, 25.573718 E, 28.v.2020, I. Remoundou. GoogleMaps 10 larvae (on 5 microscope slides, 6 NHMF: accessions GBIFCH01352028 , GBIFCH01352029 , GBIFCH01352030 , 4 SMNHTAU: accessions 427728 , 427729 ) GoogleMaps ; 2♁, 1♀ ( NHMF: accession GBIFCH01352025 , GBIFCH01352026 , GBIFCH01352027 ) ; 2♁ 1♀, ( SMNHTAU: accessions 427726 , 427727 , 416127 ), emerged between 31.iii.2021 and 02.iv.2021 . Other material: 3 pupal exuviae on two microscope slides, Greece, Crete, Katharo plateau, 35.144263 N, 25.573718 E, collected on 28.v.2020 by I. Remoundou; excised from soil on 11.xi.2021 by L. Fazan ( SMNHTAU) GoogleMaps .
Comments. The first and only previous known mention, description and drawing of the galls of this insect come from an unsigned and undated note in French inserted in the collection of the Herbarium of the University of Montpellier (MPU) (Supplementary material S1). The note is accompanied by a few flowering shoots of Z. abelicea , with leaves, fruit and galls contained in an envelope. This note is thought to have been written by the French botanist Jules Émile Planchon (1823–1888), and mentions that the galls come from Z. abelicea samples collected by the German botanist Theodor von Heldreich (1822–1902), giving a morphological description of the galls and stating that no insects were found within. No samples collected by von Heldreich are currently included in the herbarium of MPU, but samples collected by him in June 1846 which contain galls are found in the collections of other herbaria, e.g., the Conservatoire et Jardin botaniques de la Ville de Genève (G), the Muséum national d’Histoire naturelle of Paris (P), the Royal Botanic Garden of Edinburgh (RBGE), and the University of Florence (FI). J. E. Planchon included his observations of the galls in his description of Z. abelicea found in the Prodromus Systematis Naturalis Regni Vegetabilis of de Candolle ( Planchon 1873).
Recent studies ( Fazan et al. 2023) have shown that the presence of C. ampelitsiae on flowering shoots of Z. abelicea significantly reduces the number of fruits produced per shoot and fruit weight, probably due to shifts in resource allocation. Seed sterility issues are of major concern over parts of the range of Z. abelicea . However, no significant effect of the presence of C. ampelitsiae on the overall seed sterility of the trees has been found.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |