Vultocinidae
|
publication ID |
https://doi.org/ 10.5281/zenodo.178296 |
|
DOI |
https://doi.org/10.5281/zenodo.6250886 |
|
persistent identifier |
https://treatment.plazi.org/id/03898795-FFC6-FFAC-EEA2-FD9B9FD8F813 |
|
treatment provided by |
Plazi |
|
scientific name |
Vultocinidae |
| status |
|
Vultocinidae View in CoL , new family
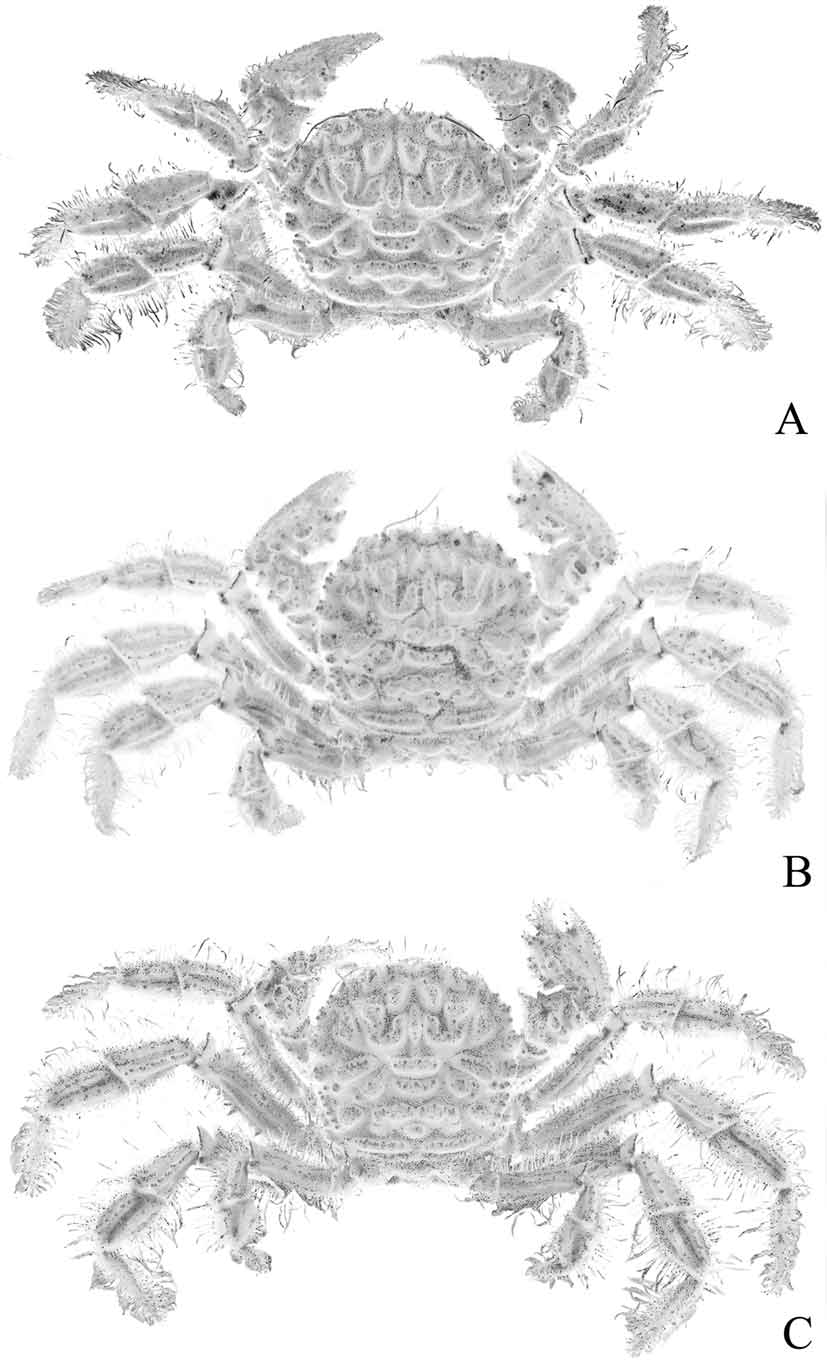
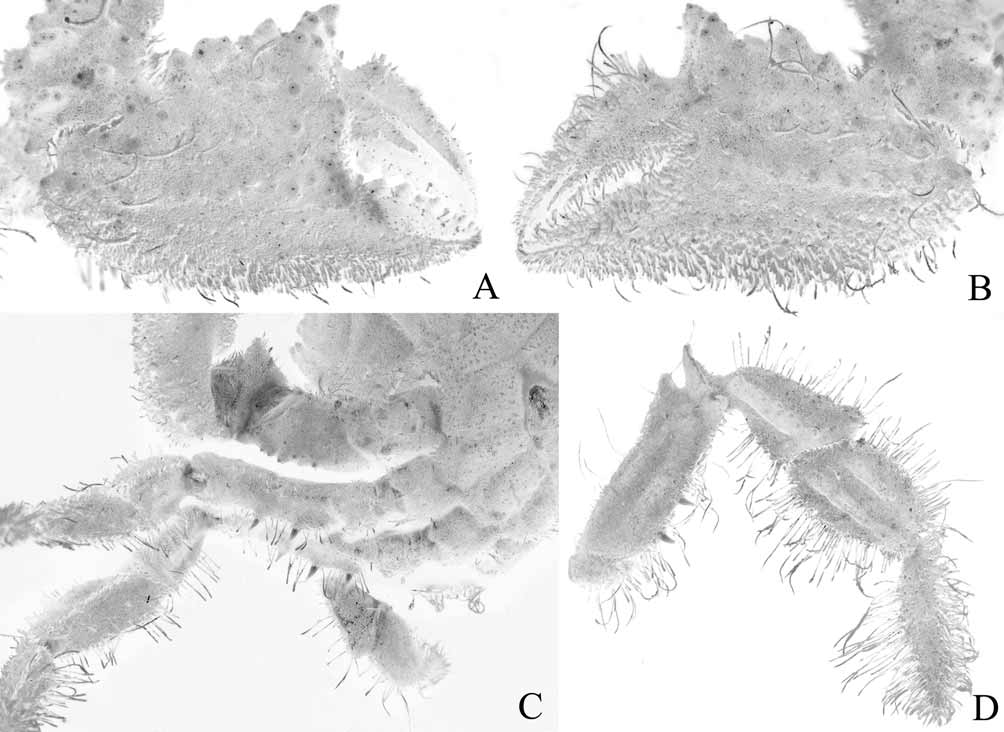
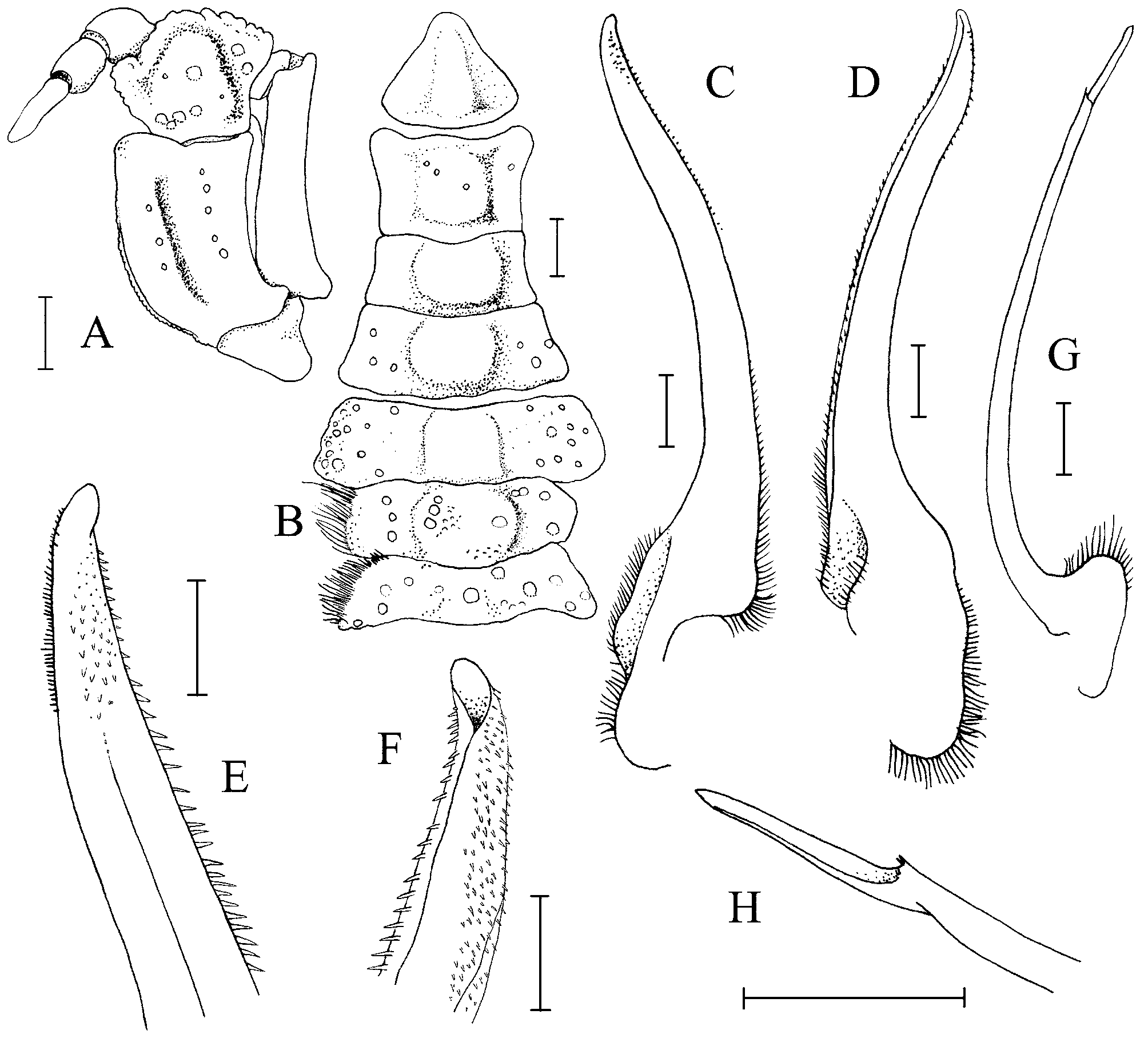
Diagnosis. Dorsal surface of carapace subquadrate, with complex pattern of ridges, grooves, dense short setae ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 A). Mandibles prominently dark coloured even after prolonged preservation ( Fig. 8 View FIGURE 8 A). Thoracic sternum relatively narrow transversely ( Fig. 9 View FIGURE 9 A); episternites 4–6 not prominently expanded laterally ( Figs. 9 View FIGURE 9 A, 10A, 12A); sternite 7 very narrow longitudinally, tapering abruptly ( Fig. 10 View FIGURE 10 A), posterior part of episternite 7 prominently prolonged backwards to form long spur which covers penis and just touches coxa of P5 ( Figs. 13 View FIGURE 13 A, B); sutures 4/5 and 5/6 prominently interrupted medially ( Fig. 12 View FIGURE 12 A); suture 6/7 appears complete but with median portion very shallow but discernible ( Fig. 12 View FIGURE 12 A); suture 7/8 distinctly complete with median part deep ( Fig. 12 View FIGURE 12 A); sternite 8 not exposed when abdomen closed ( Fig. 10 View FIGURE 10 A). Ventral margins of meri of P2– P4 with 2 or 3 strong perpendicular spines ( Fig. 4 View FIGURE 4 C, D). Sterno-abdominal cavity almost reaches anterior edge of sternite 4 ( Figs. 9 View FIGURE 9 A, 12A); press button of male abdominal locking mechanism distinct, on posterior edge of thoracic sternite 5 ( Fig. 12 View FIGURE 12 A). Penis soft, effectively coxal, exits at base of coxa of P5 but covered by posterior prolongation of episternite 7 ( Figs. 13 View FIGURE 13 A, B). Male abdomen relatively narrow overall ( Figs. 3 View FIGURE 3 B, 5B, 9A, 10A), sutures for all somites and telson clearly visible; somite 1 transversely and longitudinally broad, trapezoidal in shape, exposed, not covered by posterior carapace margin ( Fig. 11 View FIGURE 11 A), somites 2–6 relatively narrow transversely, somite 6 as long as broad ( Figs. 9 View FIGURE 9 A, 10A), somites 3, 4 immovable, fused, other somites freely articulating ( Fig. 5 View FIGURE 5 B). G1 relatively slender, gently curved, surface with simple spines ( Fig. 5 View FIGURE 5 C-F); G2 with short distal segment, about 0.2 times length of basal segment ( Fig. 5 View FIGURE 5 G, H).
Type genus. Vultocinus , new genus.
Remarks. Vultocinus , new genus, possesses a suite of unusual characters that make its precise affinities difficult to ascertain. Superficially, its carapace shape and setation pattern resembles species of Lophoplax Tesch, 1918 , but members of this genus have slender and sinuous G1s with a very short sigmoid G2, and are currently classified in the subfamily Rhizopinae Stimpson, 1858 , family Pilumnidae (see Takeda 1977; Ng & Davie 1991; Ng & Huang 2002; review by Ng 1987). The general carapace shape and setose surfaces of Vultocinus also resembles members of the genus Planopilumnus Balss, 1933 , of the subfamily Planopilumninae Serène, 1984, family Pseudoziidae ; but members of this family have stout G1s and a G2 which is only half the length of the G1 (see Ng & Clark 2000a, b; Ng et al. 2001; Ng & Liao 2002).
The possession of male abdominal somites 2 and 3 which are immovable, of a long and relatively stout G1 and of a long G2 which is subequal in length to the G1, appears to ally Vultocinus with some members of the Goneplacidae MacLeay, 1838 sensu lato. While the taxonomy of this family has been a major problem for many years (see Guinot & Breton 2006), recent studies which built on the key contributions made by Guinot (1969a – c, 1971) have made the systematic position of the taxon much clearer. This is especially so when using the suite of many new characters pioneered by her over the years (see Guinot 1979; Guinot & Bouchard 1998). Over the years, many taxa and subfamilies have been transferred from the Goneplacidae into other families (see Castro, in press, for review) and until 2003, the Goneplacidae was believed to be composed of five subfamilies, viz. the Goneplacinae MacLeay, 1838, Carcinoplacinae H. Milne Edwards, 1852, Euryplacinae Stimpson, 1871, Chasmocarcininae Serène, 1964 , and Trogloplacinae Guinot, 1986 (see Ng et al. 2001; Davie 2002). Recent studies have substantially changed the landscape of goneplacid taxonomy – Karasawa & Kato (2003a, b), Števčić (2005), Karasawa & Schweitzer (2006) and Castro (in press); which are discussed individually below. These discussions are not always restricted to the extant fauna, a major shortcoming since there is often a need to use many important characters like male abdominal structure, as well as detailed sternal and penial features which are rarely observable in fossils.
Karasawa & Kato (2003a, b) reappraised the phylogeny of the Goneplacidae and rearranged the subfamilial system based on selected extant and fossil taxa. They synonymised the Carcinoplacinae under the Goneplacinae, and also established a new subfamily, Mathildellinae, for Beuroisia Guinot & Richer de Forges, 1981, Intesius Guinot & Richer de Forges, 1981, Mathildella Guinot & Richer de Forges, 1981, Platypilumnus Alcock, 1894 , and Neopilumnoplax Serène, 1969 , which had originally been placed in the Carcinoplacinae . Although Karasawa & Kato (2003) define the Mathildellinae with a suite of carapace characters, one of the most diagnostic characters (but unfortunately only usable for fresh specimens) is whether somites 3 and 4 are mobile. All known extant adult male goneplacines (and carcinoplacines) have somites 3 and 4 freely mobile (with one exception, Neommatocarcinus Takeda & Miyake, 1969 , which has somites 3–5 fused, see also Castro, in press). On the other hand, all extant adult male mathildellines have abdominal somites 3 and 4 immobile although the sutures separating them are still visible (see also Ng & Chan 2000; Ng & Ho 2003; Crosnier & Ng 2004). The articulation between somites 4 and 5 is usually more difficult to ascertain. In the mathildellines we have examined, there is much more limited movement between these somites compared to other somites.
In a major reappraisal, Števčić (2005) substantially reorganized the family but with minimal or no discussion. He restricted what he defined as the superfamily Goneplacoidea to just three families: Goneplacidae (with two subfamilies, Goneplacinae and Carcinoplacinae , with two tribes—Carcinoplacini, Psopheticini Števčić, 2005), Geryonidae Colosi, 1923 (with three subfamilies, Geryoninae with six tribes—Geryonini, Progeryonini Števčić, 2005, Paragalenini Števčić, 2005, Mathildellini, Intesiini Števčić, 2005, Platycheloniini Števčić, 2005; Platypilumninae Števčić, 2005; and Bathyplacinae Števčić, 2005 ), and Planopilumnidae (as Planopilumninae [sic]) Serène, 1984. Many other taxa which had been placed in the Goneplacidae up to 2005 were transferred to other groups or whole new families and subfamilies. His recognition of some of them as families, e.g. the Euryplacidae and Chasmocarcininae as distinct families was expected as there had been much prior discussion on their status, although his unexplained elevation of both groups to superfamily status seems unwarranted. The same applies for placing Scalopidia Stimpson, 1858 , into its own family, the Scalopidiidae Števčić, 2005 , in the superfamily Chasmocarcinoidea. The subfamily Trogloplacinae Guinot, 1986 (regarded as a family by Davie 2002), has clear chasmocarcinid affinities (see Guinot & Davie 1996), but this was ignored and Števčić (2005) recognised it as a distinct superfamily, separate from his Chasmocarcinoidea. Two genera which had been allied to the Chasmocarcinidae , Parapilumnus Kossmann, 1877 , and Acidops Stimpson, 1871 , were inexplicably referred to their own families (Parapilumnidae Števčić, 2005, and Acidopsidae Števčić, 2005 , respectively) and superfamilies in two different parts of his paper, although the affinities between them are very clear and should be classified together ( Ng 2002). Two other genera linked with the Chasmocarcinidae , Raoulia Ng, 1987 , and Typhlocarcinodes Alcock, 1900 , were also referred to their own families (Raoulidae Števčić, 2005, and Typhlocarcinodidae Števčić, 2005, respectively), and own superfamilies. One genus clearly affiliated with the Goneplacidae and Goneplacinae sensu lato, Notonyx A. Milne- Edwards, 1873, was removed to its own family (Notonycidae Števčić, 2005) and superfamily Notonycoidea Števčić, 2005 (see also Clark & Ng 2005; Castro in press). The genus Conleyus Ng & Ng, 2003 , originally placed in the Carcinoplacinae , was refered to its own family ( Conleyidae Števčić, 2005 ). Some of his actions are very difficult to rationalize, e.g. Parapilumoidea and Acidoposidea, even if both are not true chasmocarcinids as defined by Guinot & Davie (1996). The same situation prevails for Raoulia and Typhlocarcinodes . Both genera may not be chasmocarcinids, but to classify them as belonging to separate superfamilies is difficult to understand. The case with Notonyx and Notonycoidea also suggests that Stevčić (2005) placed too much emphasis on general external morphology. While Conleyus has many peculiar features, the need for a separate superfamily is also unwarranted because the genus has many affinities with goneplacids. Stevčić’s (2005) inclusion of Geryonidae and Planopilumnidae into his Goneplacoidea is also difficult to understand. This is also true for his transfer of the Mathildellinae (as a tribe) into his Geryoninae and the removal of genera previously included in it like Intesius and Platypilumnus into its own tribe and subfamily respectively. The affinities of the geryonids with portunoids has been well argued (see Ng & Manning 1998; Ng & Guinot 1999), while planopilumnids have been included in the Pseudoziidae Alcock, 1898 by Ng et al. (2001) and Ng & Liao (2002). Intesius (and Platypilumnus ) is closely allied to Mathidella (see also Karasawa & Kato 2003a; Crosnier & Ng 2004) and separating them makes little sense. The lack of any explanation or justification by Stevčić (2005) makes these taxonomic actions and transfers difficult to accept, refute or even discuss.
Karasawa & Schweitzer (2006), in another reappraisal of the Goneplacidae , this time comparing it with the Xanthoidea, followed some aspects of Stevčić’s (2005) system, but argued that the Mathildellinae was actually a portunoid and recognized it as a separate family there. They regarded the Goneplacinae as synonymous with the Carcinoplacinae ; and placed both the Mathildellidae (as a family) and the Geryonidae in the Portunoidea. The transfer of the Mathildellinae is difficult to contend with as with regards to the general form of the carapace, sternal, abdominal and gonopodal characters, it has many affinities with the goneplacids. Other parts of their Goneplacoidea appear to be more reasonable, with the Euryplacidae , Chasmocarcinidae , Trogloplacidae and Hexapodidae Miers, 1886 . More difficult to accommodate is their transfer of the genus Progeryon Bouvier, 1922 , to its own family (i.e. the Progeryonidae Števčić, 2005 ) and superfamily which they justified stating that the form of the male thoracic sternum is different from those in other portunoids, including mathildellids. They left the matter of Paragalene Kossmann, 1878 , and the Paragaleninii Števčić, 2005, unresolved,
Most recently, Castro (in press) reviewed and revised the members of the species which have been placed in Goneplacinae MacLeay, 1838, and Carcinoplacinae H. Milne Edwards, 1852, and supported Karasawa & Kato’s (2003) synonymisation of these two subfamilies. He also recognised the Mathildellinae as a separate subfamily within the Goneplacidae , and synonymised Notonycidae under the Goneplacinae. As to the status of genera like Bathyplax A. Milne-Edwards, 1880 , Progeryon , Paragalene , Conleyus , etc., he argued that they were not certainly not goneplacines in the strict sense, but may perhaps be retained in the Goneplacidae sensu lato provisionally.
How does Vultocinus fit into all these recent developments? The problem is that many of the subfamilies or families defined by the earlier workers have omitted key characters associated with the thoracic sternum, abdominal locking mechanism, position of the penis and associated structures. The most comprehensive study (Castro, in press) has many of the characters that are deemed useful in a modern classification but it is mostly restricted to the Goneplacinae sensu stricto, although he does briefly discuss many of the other “goneplacid allies”. To resolve the systematic position of Vultocinus , it was important that representative genera of the various supra-generic groupings discussed above be re-examined. To this effect, we examined in detail representative genera from the Goneplacinae s. str., Mathildellinae s. str., as well as two of the problematic genera, Progeryon and Conleyus , and compared them with Vultocinus .
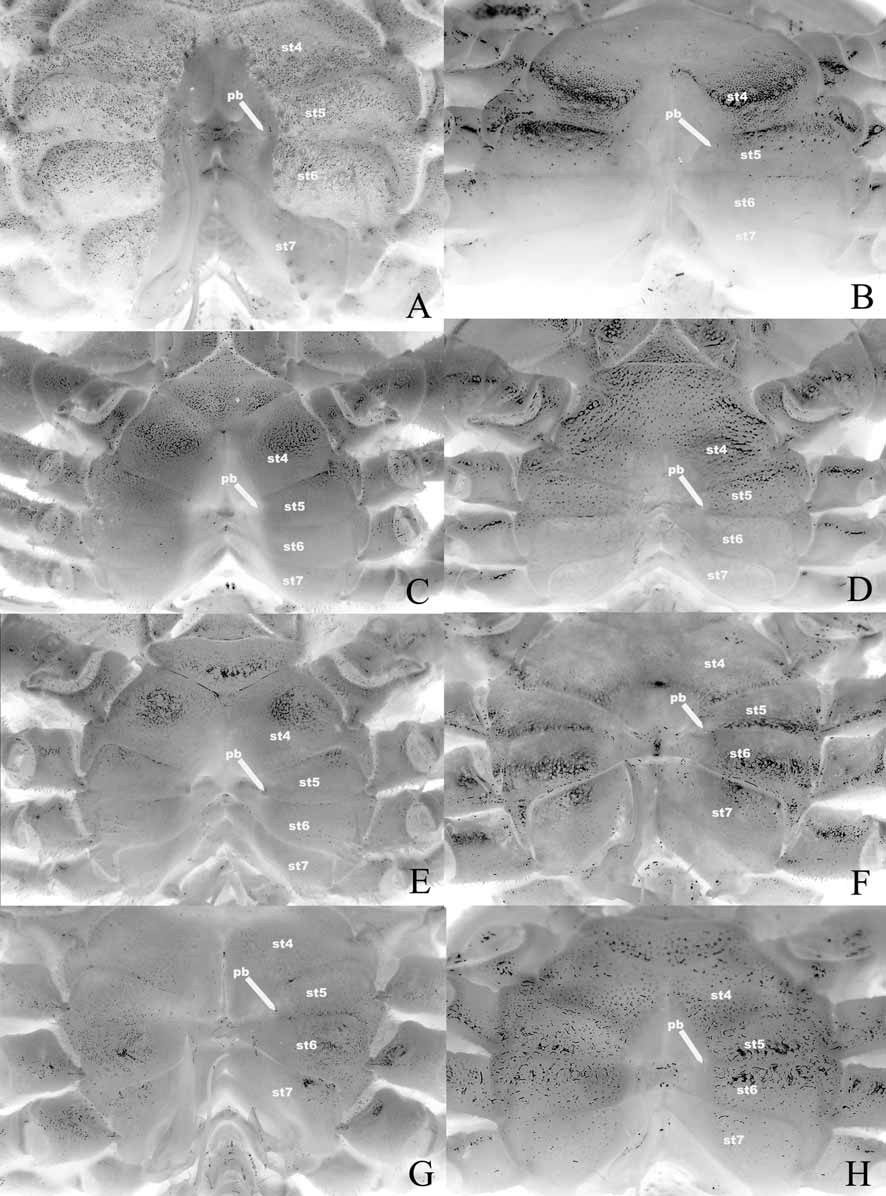
The position of the press button of the abdominal locking mechanism (see Guinot & Bouchard 1998) on or along thoracic sternite 5 is important. While all of the genera considered here have the button on thoracic sternite 5 at the lateral edges of the sterno-abdominal cavity,there are two distinct patterns. In one group with Goneplax Leach, 1814 , and Carcinoplax H. Milne Edwards, 1852 , the press button is on the anterior part of sternite 5, close to sternite 4 ( Fig. 12 View FIGURE 12 B, C). In all the other genera— Mathildella , Intesius , Platypilumnus , Progeryon , Conleyus and Vultocinus , the press button is on the posterior part of sternite 5, close to sternite 6 ( Fig. 12 View FIGURE 12 A, D–H). This correlates somewhat with the extent of the sterno-abdominal cavity in some cases. In Goneplax and Carcinoplax , the sterno-abdominal cavity is usually relatively deep, and extends almost to the anterior edge of thoracic sternite 4 ( Figs. 9 View FIGURE 9 B, C, 12B, C). In Mathildella , Intesius and Platypilumnus , the sterno-abdominal cavity is usually relatively shallow, and extends only to the middle part of thoracic sternite 4 ( Figs. 9 View FIGURE 9 D–F, 12D–F). The position of the sterno-abdominal cavity in Progeryon , however, is like that of Goneplax and Carcinoplax , almost reaching the anterior edge of sternite 4 ( Fig. 9 View FIGURE 9 G) but the cavity is relatively shallower ( Fig. 12 View FIGURE 12 G). The position of the sterno-abdominal cavity in Conleyus is intermediate between that of Goneplax , Carcinoplax and Progeryon on one hand, and Mathildella , Intesius and Platypilumnus on the other ( Fig. 9 View FIGURE 9 H), although the cavity itself is relatively deep ( Fig. 12 View FIGURE 12 H). Vultocinus stands out in that it has a deep sterno-abdominal cavity which is relatively narrow transversely ( Fig. 12 View FIGURE 12 A) and almost reaches the anterior edge of sternite 4 ( Fig. 9 View FIGURE 9 A).
A note on the use of the extent of the sterno-abdominal cavity as a character is useful. In some taxa, the anterior-most point of the cavity extends beyond the tip of the telson, even when the male abdomen is fully closed and locked into position by the press button mechanism. It is most distinct in genera like Goneplax , Carcinoplax and Vultocinus ( Fig. 9 View FIGURE 9 A–C), and to a lesser extent by genera like Progeryon and Conleyus ( Fig. 9 View FIGURE 9 G, H). It is not so obvious in Mathildella , Intesius and Platypilumnus ( Fig. 9 View FIGURE 9 D–F) because the sterno-abdominal cavity is relatively shallower and the male abdomen always locks onto the sternum very tightly. The gap is presumably to enable the animals to defecate without moving the rest of the abdomen or even the telson (see also Guinot & Bouchard 1998).
The structure of the sutures 6/7 and 7/8 are useful in understanding the relationships between the groups. In the two species of Goneplax examined in this study, suture 6/7 can be regarded as complete even though it becomes rather shallow medially and appears interrupted ( Fig. 12 View FIGURE 12 B). In Carcinoplax , the same suture also appears to be complete but there is a narrow median gap ( Fig. 12 View FIGURE 12 C) and so may be regarded as interrupted. In the case of Vultocinus , this suture curves anteriorly, becoming very shallow medially so it is not as easy to ascertain if this is truly complete or a case of incipient interruption ( Fig. 12 View FIGURE 12 A). In the Goneplacinae sensu Castro (in press), suture 6/7 varies between complete and interrupted. In all the other genera examined here, suture 6/7 is clearly complete ( Fig. 12 View FIGURE 12 D–H). With regards to suture 7/8, in Goneplax , it is prominently interrupted medially ( Fig. 12 View FIGURE 12 B). In Carcinoplax , the suture appears to be complete but there is a narrow median interruption ( Figs. 7 View FIGURE 7 E, 12C) and so may be regarded as interrupted as well. According to Castro (in press), while most Goneplacinae are like Goneplax and Carcinoplax , there some taxa which have suture 7/8 complete. In all the other genera examined here (including Vultocinus ), suture 7/8 is clearly complete ( Fig. 12 View FIGURE 12 A, D–H).
Also of interest is the arrangement of thoracic sternite 8 in relation to the adjacent coxa of P5 and male abdomen. Guinot (1969b: 520) first highlighted this interesting character in her attempts to better understand goneplacoid relationships. In genera like Goneplax and Carcinoplax , part of sternite 8 is still clearly visible even after the abdomen is completely closed. In species of Goneplax sensu stricto, the exposed part of sternite 8 is quite substantial ( Fig. 7 View FIGURE 7 C) (see also Guinot 1969b: Figs. 63, 64). In at least some of the Indo-West Pacific species of “ Goneplax ”, the exposed part of sternite 8 is relatively small ( Figs. 10 View FIGURE 10 B, 11b), while in genera like Carcinoplax , the exposed part of sternite 8 varies from small ( Figs. 10 View FIGURE 10 C, 11C) to relatively large (see Guinot 1969b: Figs. 60–62, 65). In genera like Mathildella , Intesius and Platypilumnus , the exposed part of sternite 8 is either completely covered by the abdomen ( Figs. 10 View FIGURE 10 D, F, 11D, F) or just visible with only a small part of the edge visible ( Figs. 10 View FIGURE 10 E, 11E). In Progeryon and Conleyus , the lateral part of sternite 8 completely covered by the male abdomen ( Figs. 10 View FIGURE 10 G, H, 11G, H).
The position of the penis is of fundamental importance (Guinot, D., pers. comm.). This character is closely associated with the shape of episternite 7 and the lateral margin of abdominal somite 3. In all cases, the exposed part of the penis is protected by abdominal somite 3 when the abdomen is closed. How this is accomplished is what distinguishes each of the groups.
In the Goneplacidae sensu stricto, the penis leaves the coxa at its base, but continues under the sternum for a short distance, only exiting and becoming completely exposed inside the sterno-abdominal cavity after the coxal condyle ( Fig. 13 View FIGURE 13 C, D). Episternite 7 is expanded laterally to partially overlap the entire inner margin of the coxa of P5 ( Figs. 7 View FIGURE 7 D, 10B, 13C) or at least most of the anterior part ( Figs. 10 View FIGURE 10 C, 12D). The lateral edge of abdominal somite 3 never reaches the coxa of P5, at most touching the condyle ( Figs. 7 View FIGURE 7 D, 10B, C). In Goneplax , the penis is clearly of the coxosternal type, exiting prominently between sternites 7 and 8 at a submedially position ( Figs. 7 View FIGURE 7 F, 13C). In Carcinoplax , the penis exits just after the condyle, at the beginning of the junction between sternites 7 and 8 ( Fig. 13 View FIGURE 13 D). As a result, the distal part of the penis, where it comes out of the coxa, is protected by the expanded part of episternite 7, and the exposed part of the penis, where it exits between sternites 7 and 8, is protected by the closed abdomen.
The same situation is true for Conleyus , i.e. the penis is clearly coxosternal, exiting between sternites 7 and 8 at a submedian position ( Fig. 13 View FIGURE 13 F), episternite 7 is expanded laterally to partially overlap most of the anterior part ( Fig. 10 View FIGURE 10 H) and the lateral edge of male abdominal somite 3 does not reach the coxa of P5 or its condyle ( Figs. 10 View FIGURE 10 H, 11F). The penis is therefore partially protected by the expanded episternite 7 and the closed abdomen.
In Mathildella , Intesius and Progeryon , the penis is clearly coxal, opening near the condyle of the coxa of P5 ( Fig. 13 View FIGURE 13 E), episternite 7 is not expanded laterally to any major degree and either does not reach the coxa of P5 ( Fig. 10 View FIGURE 10 D) or only just touches the anterior part ( Fig. 10 View FIGURE 10 E–G), and the lateral edge of male abdominal somite 3 covers the inner part of the coxa of P5, including the condyle ( Fig. 10 View FIGURE 10 D–G). In such an arrangement, the exposed part of the penis is protected almost completely by the abdomen.
The configuration in Vultocinus is unique. The penis is clearly coxal in origin, opening near the condyle of the coxa of P5 ( Fig. 13 View FIGURE 13 A, B) and the lateral edge of male abdominal somite 3 covers the inner part of the coxa of P5 but not the condyle ( Fig. 10 View FIGURE 10 A). Instead, the condyle of the coxa of P5 is partially covered by the expanded lateral margin of abdominal somite 2 ( Fig. 10 View FIGURE 10 A). The posterior edge of episternite 7 is very unusual in that is strongly produced posteriorly to form a spur-like projection which partially covers the anterior edge of the coxa of P5 and brackets the exposed penis ( Fig. 13 View FIGURE 13 A, B). The penis is therefore protected laterally by the strongly produced episternite 7 and the closed abdomen. Because of this, the penis of Vultocinus can be described as coxosternal.
Looking at these datasets altogether (Table 1), there seems little doubt that Vultocinus needs to be referred to its own family, Vultocinidae . Its combination of male sternal, abdominal and penial structures in particular, is unique. The data sets also strongly indicate that the subfamily Mathildellinae is quite distinct from the Goneplacinae (as defined by Castro, in press), and should be regarded as a distinct family as has been suggested by Karasawa & Schweitzer (2006), although we do not agree with its placement in the Portunoidea. The Goneplacidae sensu stricto therefore now has just two subfamilies, Goneplacinae sensu stricto, and Bathyplacinae Števčić, 2005 (see discussion below). We also agree with Karasawa & Schweitzer (2006) in recognizing the Progeryonidae as a distinct family, but certainly not at the superfamily level. The differences cited by them are not valid, but other characters discussed here suggest that it should not be placed in the Goneplacidae as presently defined. For convenience, in lieu of examining specimens of Paragalene , we also keep this genus with Progeryon in the Progeryonidae . The data sets also argue for Conleyus to be in its own family, the Conleyidae , as has been done by Števčić (2005) although the reasons cited by him (including for his superfamily) are mostly invalid. Števčić (2005) did not examine material and had relied entirely on the original description by Ng & Ng (2003) who did not mention or detail any of the key characters presented in this paper. As with the case of many of Števčić’s (2005) suprageneric taxa, they have been effectively vindicated here by default, even though the characters originally cited by him as justification for these new taxa are incorrect. As a summary, the families may be briefly diagnosed as follows, the ones for Goneplacidae sensu stricto, and Mathildellidae sensu stricto, augmenting those by Castro (in press) for these two taxa.
Diagnosis of Goneplacidae MacLeay, 1838 : Dorsal surface of carapace smooth to setose, regions usually discernible but never very prominent or with deep grooves ( Fig. 6 View FIGURE 6 A–C). Mandible white to grey in life and preserved ( Figs. 7 View FIGURE 7 A, 8B). Thoracic sternum relatively broad to very broad; episternites 4–7 expanded laterally to form broad plate which covers anterior edge of coxa of P5 ( Figs. 7 View FIGURE 7 B, D, 9B, C, 10B, C, 12B, C, 13C, D); surface of sternite 3 without longitudinal median line ( Figs. 7 View FIGURE 7 B, 9B, C); sternite 7 broad to very broad ( Figs. 7 View FIGURE 7 D, 10B, C); sutures 4/5 and 5/6 interrupted medially ( Figs. 7 View FIGURE 7 E, 12B, C); sutures 6/7 complete and 7/8 distinctly interrupted medially ( Fig. 7 View FIGURE 7 E), sutures 6/7 and 7/8 with small gap medially suggesting interruption ( Fig. 12 View FIGURE 12 B, C), or occasionally complete; lateral part of sternite 8 distinctly exposed when male abdomen closed, visible as a large plate between coxa and abdomen ( Fig. 7 View FIGURE 7 D) or a small rounded or ovate plate ( Fig. 10 View FIGURE 10 B, C). Ventral margins of meri of P2–4 unarmed or only with spinules or granules ( Fig. 6 View FIGURE 6 A-C). Sternoabdominal cavity almost reaches to anterior edge of sternite 4 ( Figs. 7 View FIGURE 7 B, 9B, C, 12B, C); press button of male abdominal locking mechanism on anterior edge of sternite 5 ( Figs. 7 View FIGURE 7 E, 12B, C). Penis coxosternal, originating from coxa of P5 but exiting between sternites 7 and 8, beyond condyle of coxa ( Figs. 7 View FIGURE 7 F, 13C, D). Male abdomen broadly triangular ( Figs. 7 View FIGURE 7 B, D, 9B, C, 10B, C), sutures for all somites and telson clearly visible, somite 1 transversely broad but very narrow longitudinally, sometimes partially hidden under posterior carapace margin ( Figs. 7 View FIGURE 7 C, 11B, C), somites 2–6 prominently transversely broad, somite 6 distinctly broader than long ( Figs. 7 View FIGURE 7 B, D, 9B, C, 10B, C), all somites freely mobile. G1 relatively straight or slightly curved, sinuous, slen- der to stout, broad basal part gradually tapering distally, tip usually dilated/truncated. G2 usually as long as G2 (occasionally much shorter in a few genera), distal segment usually long, as long as, subequal to or longer than basal segment (sometimes short). Constituent genera: Goneplax Leach, 1814 , Carcinoplax H. Milne Edwards, 1852 , Notonyx A. Milne-Edwards, 1873 , and genera listed in Castro (in press) for his Goneplacinae.
Diagnosis of Mathildellidae Karasawa & Kato, 2003 a: Dorsal surface of carapace smooth to setose, regions sometimes distinct but never prominent, grooves shallow ( Fig. 6 View FIGURE 6 D–F). Mandibles white to grey in life and preserved ( Fig. 8 View FIGURE 8 B). Thoracic sternum usually broad, sometimes relatively narrower; episternites 4–6 expanded laterally to some degree or other, never prominently ( Figs. 9 View FIGURE 9 D–F, 10D–F, 12D–F); episternite 7 not expanded laterally, neither touching coxa of fourth ambulatory leg nor forming small plate which partially covers or just touches anterior edge of coxa of P5 ( Fig. 13 View FIGURE 13 E); surface of sternite 3 without longitudinal median line ( Fig. 9 View FIGURE 9 D–F), sternite 7 relatively broad to somewhat narrow transversely ( Fig. 10 View FIGURE 10 D–F); sutures 4/5 and 5/ 6 interrupted medially ( Fig. 12 View FIGURE 12 D–F); sutures 6/7 and 7/8 complete, median part of suture especially deep, well demarcated ( Fig. 12 View FIGURE 12 D–F); lateral part of sternite 8 completely covered by closed male abdomen ( Fig. 10 View FIGURE 10 D, F) or barely visible with only the edge visible ( Fig. 10 View FIGURE 10 E). Ventral margins of meri of P2–4 usually smooth or granulated ( Fig. 6 View FIGURE 6 D, E), rarely spiniform (except for Platypilumnus , Fig. 6 View FIGURE 6 F) but if so, not like condition in Vultocinidae . Sterno-abdominal cavity reaches to middle part of sternite 4, never approaching median area ( Figs. 9 View FIGURE 9 D–F, 12D–F); press button of male abdominal locking mechanism on posterior edge of sternite 5 ( Fig. 12 View FIGURE 12 D–F). Penis coxal, originating directly from base of coxa of P5 ( Fig. 13 View FIGURE 13 E). Male abdomen triangular ( Figs. 9 View FIGURE 9 D, E, 10D, E) but may be somewhat quadrate (e.g. Platypilumnus , Figs. 9 View FIGURE 9 F, 10F), sutures for all somites and telson clearly visible, somite 1 transversely broad but relatively narrow longitudinally, subrectangular in shape to somewhat sinuous, exposed, not covered by posterior carapace margin ( Fig. 11 View FIGURE 11 D–F), somites 2–6 distinctly transversely broad, somite 6 distinctly broader than long ( Figs. 9 View FIGURE 9 D–F, 10D–F), somites 3 and 4 immovable although suture evident, somite 4 and 5 sometimes immobile as well. G1 C-shaped, curving outwards, basal part very broad, abruptly tapering to slender median and distal parts. G2 as long as G1, distal segment long, two-thirds to three-quarters length of basal segment. Constituent genera: Mathildella Guinot & Richer de Forges, 1981, Beuroisia Guinot & Richer de Forges, 1981, Intesius Guinot & Richer de Forges, 1981, Platypilumnus Alcock, 1894 , Neopilumnoplax Serène, 1969 .
Diagnosis of Progeryonidae Štev ě i ć, 2005: Dorsal surface of carapace with very short felt-like setae, regions discernible but low, grooves shallow ( Fig. 6 View FIGURE 6 G). Mandibles white in preservative ( Fig. 8 View FIGURE 8 C). Thoracic sternum relatively broad; episternites 4–6 not prominently expanded laterally ( Figs. 9 View FIGURE 9 G, 10G, 12G); episternite 7 not prominently expanded but with small plate which just touches anterior edge of coxa of P5 (as in Fig. 13 View FIGURE 13 E); sternite 3 without longitudinal median line ( Fig. 9 View FIGURE 9 G); sternite 7 very narrow transversely, tapering gradually posteriorly ( Fig. 10 View FIGURE 10 G); sutures 4/5 and 5/6 interrupted medially but median grooves very shallow ( Fig. View FIGURE 12
12G); sutures 6/7 and 7/8 complete, median part of suture especially deep, well demarcated; lateral part of sternite 8 completely covered by closed male abdomen ( Fig. 10 View FIGURE 10 G). Ventral margins of meri of P2–4 gently granulated ﹝Fiɡ․ ₆G). Sterno-abdominal cavity almost reaches anterior edge of sternite 4 ( Figs. 9 View FIGURE 9 G, 12G); press button of male abdominal locking mechanism on posterior edge of sternite 5 ( Fig. 12 View FIGURE 12 G). Penis coxal, originating directly from base of coxa of P5 (as in Fig. 13 View FIGURE 13 E). Male abdomen appears subovate due to broad somites and distinctly convex margins ( Figs. 9 View FIGURE 9 G, 10G), sutures for all somites and telson clearly visible, somite 1 relatively narrow longitudinally but also relatively narrow transversely, sublunate in shape, completely exposed, not covered by posterior carapace margin ( Fig. 11 View FIGURE 11 G), somites 2–6 distinctly transversely broad, somite 6 much broader than long ( Figs. 9 View FIGURE 9 G, 10G), all somites 3 mobile. G1 relatively stout, almost straight, gradually tapering to relatively sharp tip. G2 prominently longer than G1, distal segment long, almost as long as basal segment. Constituent genera: Progeryon Bouvier, 1922 , Paragalene Kossmann, 1878 .
Diagnosis of Conleyidae Štev ě i ć, 2005: Dorsal surface of carapace with poorly defined regions and shallow grooves, sparsely setose ( Fig. 6 View FIGURE 6 H). Mandibles white in life and preserved ( Fig. 8 View FIGURE 8 D). Thoracic sternum broad; episternites 4–6 expanded laterally slightly ( Figs. 9 View FIGURE 9 H, 10H, 12H); episternite 7 distinctly expanded laterally to form plate which covers anterior edge of coxa of P5 ( Fig. 13 View FIGURE 13 F); sternite 3 without longitudinal median line ( Fig. 9 View FIGURE 9 H); sternite 7 relatively broad ( Fig. 10 View FIGURE 10 H); sutures 4/5 and 5/6 interrupted medially ( Fig. 12 View FIGURE 12 H); sutures 6/7 and 7/8 complete (as in Mathildellidae ), suture more or less evenly prominent along length ( Fig. 12 View FIGURE 12 H); lateral part of sternite 8 completely covered by closed male abdomen ( Fig. 10 View FIGURE 10 H). Ventral margins of meri of P2–4 granuliform ( Fig. 6 View FIGURE 6 H). Sterno-abdominal cavity reaches to middle part of sternite 4 ( Figs. 9 View FIGURE 9 H, 12H); press button of male abdominal locking mechanism on posterior edge of sternite 5 ( Fig. 12 View FIGURE 12 H). Penis coxosternal, originating from coxa of P5 but exiting between sternites 7 and 8, beyond condyle of coxa ( Fig. 13 View FIGURE 13 F). Male abdomen triangular ( Figs. 9 View FIGURE 9 H, 10H), sutures for all somites and telson clearly visible, somite 1 transversely broad but narrow longitudinally, sinuous, exposed, not covered by posterior carapace margin ( Fig. 11 View FIGURE 11 H), somites 2–6 transversely broad, somite 6 broader than long ( Fig. 9 View FIGURE 9 H, 10H), all somites mobile although somites 3 and 4 somewhat stiff but still movable. G1 relatively slender, sinuous, with tip directed upwards. G2 as long as G1, distal segment short, about 0.3 times length of basal segment. Constituent genus: Conleyus Ng & Ng, 2003 .
As to their superfamily status, we retain the Mathildellidae , Progeryonidae , Conleyidae and Vultocinidae in the Goneplacoidea for the time being. As for some of the other unusual suprageneric taxa established or recognized by Števčić (2005) discussed above, viz. Psopheticini Števčić, 2005, Platypilumninae Števčić, 2005, Bathyplacinae Števčić, 2005 , Scalopidiidae Števčić, 2005 , Platycheloniini Stevcic, 2005, and Planopilumnidae Serène, 1984 ; the following decisions are taken. Psopheticini Števčić, 2005, is clearly a synonym of Goneplacinae sensu stricto (see Castro, in press). The Platypilumninae is a synonym of the Mathildellidae (see above). The Bathyplacinae has several unusual features and should be tentatively recognized as a subfamily of the Goneplacidae (see above). The Scalopidiidae is probably also a valid family, but may be allied to the Chasmocarcinidae (D. Guinot, pers. comm.). The Platycheloniini is clearly a synonym of the Planopilumnidae , and is allied to the Pseudoziidae (see Ng 2003). The systematics of the Bathyplacinae , Scalopidiidae and Pseudoziidae is currently being reappraised in detail by the first author with D. Guinot and P. Davie). All are here regarded in any case as goneplacoids.
A note on Pilumnoplax Stimpson, 1858 , is necessary. The genus was affiliated with the Mathildellinae, but is now a junior subjective synonym of Eucrate De Haan, 1835 (type species Cancer (Eucrate) crenatus De Haan, 1835 ), in the Euryplacinae Stimpson, 1871. Its type species, Pilumnoplax sulcatifrons Stimpson, 1858 , is now generally accepted as a species of Eucrate , and all the other species which have been placed in Pilumnoplax over the years have to be transferred to other genera, e.g. Neopilumnoplax Serène, 1969 (see also Tesch 1918; Guinot 1969c; Manning & Holthuis 1981).
The use of the genus Goneplax here is in the broad sense. Castro (in press) revises the genus and all of the strictly Indo-West Pacific species were transferred to new genera. Goneplax sensu stricto is restricted for Atlantic species. An exception is a new taxon here labeled as Goneplax , new species, which is closely affiliated with G. rhomboides (Linnaeus, 1758) , the type species of the genus, and currently being described by Guinot & Castro (in press). It is mostly Atlantic in distribution but it also found on the Indian Ocean coast of South Africa and hence in the Indo-West Pacific region. The Indo-West Pacific “ Goneplax ” marivenae Komatsu & Takeda, 2004, on the other hand, belongs to its own genus (Castro, in press). Both are considered and figured in this paper.
Comparative material. Goneplax , new species: male (cl 21.3 mm cw 35.3 mm) ( ZRC 2004.700), Algoa Bay, Port Elisabeth, South Africa, coll. trawlers, 1 Dec 2003; “ Goneplax ” marivenae Komatsu & Takeda, 2004: male (cl 11.0 mm, cw 17.6 mm) ( ZRC 2004.724), Balicasag Island, Panglao, Bohol, Visayas, in tangle nets, coll. local shell fishermen, 25–30 Jul 2004; Carcinoplax spinosissima Rathbun, 1914 : male (cl 24.0 mm, cw 29.1 mm) ( ZRC 2001.535), Balicasag Island, Panglao, Bohol, Visayas, in tangle nets, coll. local shell fishermen, 28 Nov 2001; Mathildella rubra Ng & Ho, 2003 : male (cl 16.2 mm, cw 19.5 mm) ( ZRC 2004.535), Balicasag Island, Panglao, Bohol, Visayas, in tangle nets, coll. local shell fishermen, 28 Nov 2003; Intesius pilosus Guinot & Richer de Forges, 1981: male (cl 32.3 mm, cw 38.3 mm) ( ZRC 2004.731), Balicasag Island, Panglao, Bohol, Visayas, in tangle nets, coll. local shell fishermen, 2 Mar 2004; Platypilumnus gracilipes Alcock, 1894 : male (cl 25.3 mm, cw 29.6 mm) ( ZRC 2004.717), Balicasag Island, Panglao, Bohol, Visayas, in tangle nets, coll. local shell fishermen, Dec 2003; Progeryon mus Ng & Guinot, 1999 : holotype male (cl 38.6 mm, cw 46.5 mm) ( ZRC 1997.442), Nihoa Island, Hawaii, 441m depth, coll. 1979; Conleyus defodio Ng & Ng, 2003 : holotype male (cl, 12.2 mm, cw 16.4 mm) ( FLMNH), Luminao fore-reef, 220 feet deep, in coral rubble, Guam, coll. H. T. Conley, Oct 1999.
TABLE 1. Differences between Vultocinidae and allies.
...... continued
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.