Bathyraja aleutica ( Gilbert, 1896 )
|
publication ID |
https://doi.org/10.11646/zootaxa.5142.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:AB36996C-74D9-416A-94C2-106345FAFF75 |
|
DOI |
https://doi.org/10.5281/zenodo.6601284 |
|
persistent identifier |
https://treatment.plazi.org/id/038987A4-9337-FFF4-73D5-FBA6CDFB0CED |
|
treatment provided by |
Plazi |
|
scientific name |
Bathyraja aleutica ( Gilbert, 1896 ) |
| status |
|
Bathyraja aleutica ( Gilbert, 1896) View in CoL
Figures 6–7 View FIGURE 6 View FIGURE 7 ; Table 2 View TABLE 2 , 8–9 View TABLE 8 View TABLE 9
Aleutian Skate
Raja aleutica Gilbert, C.H., 1896: 397 , Pl. 21 [ United States Commission of Fish and Fisheries, Report of the Commissioner] v. 19 (for 1893) (art. 6). Holotype: USNM 48548 About USNM . North of Sannak Pass , Aleutian Islands, Albatross station 3257, depth 81 fathoms.
Raja aleutica: Gilbert, 1896: 397 , pl. 21; Miller & Lea, 1972 (description, figure, key).
Breviraja aleutica: Ishiyama, 1952: 9 (key).
Bathyraja aleutica: Amaoka View in CoL , et al., 1983: 59; 170; Eschmeyer et al., 1983: 49; Masuda et al., 1984: 14; Stehmann, 1986: 263; McEachran & Dunn, 1998: 286 (listed); Dolganov, 1999: 429; Compagno, 1999: 488 (listed); Sheiko & Fedorov, 2000: 15; Mecklenburg et al., 2002 (listed): 99; Nakabo, 2002 (key, listed): 166; Hoff, 2002: 145; Ebert, 2003: 195–196 (description, distribution); Orlov, 2003: 45; Fedorov et al., 2003: 15; Nelson et al., 2004: 55; Love et al., 2005: 11; Ebert & Compagno, 2007: 116 View Cited Treatment (listed); Ebert & Davis, 2007: 4–5 View Cited Treatment (egg case description); Page et al., 2013: 55; Shinohara et al., 2014: 234 (listed); Parin et al., 2014: 30 (listed); Dyldin, 2015: 61 (listed); Weigmann, 2016: 90 (listed); Last et al., 2016: 25, 376 (listed, figure); Kells et al., 2016: 78 (figure); Ebert et al., 2017: 21, 58, 67 (description, distribution, key, listed); Dyldin & Orlov, 2018: 168; Burton & Lea, 2019: 32 (listed); Dyldin & Orlov, 2021: 58 (listed).
Diagnosis. Large, rhomboidal skates ( 1,540 mm TL), long head length (16.7–24.3% TL), and rounded pectoral apices; interdorsal space large (1.3–3.1% TL); claspers short and stubby, tip rounded and not bulbous, weakly defined pseudosiphon present, dorsal lobe has a short pseudorhipidion, U-shaped cleft, ventral lobe with a rounded projection; teeth in 34–42 and 32–38 rows on upper and lower jaw, respectively; pectoral radials 90; pelvic fins, 22; total vertebrae 152; dorsal surface with fine prickles, becoming larger on the tail; thorns present on dorsal surface, males possess alar thorns, malar thorns absent, middorsal thorns developed and high in number (4–11), nuchal thorns strong (3–5), scapular and tail thorns present, interdorsal thorns weak (1–2); dorsal coloration dark brown to grey, dark spots on pectorals, ocellus on either pectoral fin often present, ventral coloration white, with darker coloration on the snout, gills, disc margins, pelvics, and underside of the tail.
Description. A large skate with a rhomboidal disc, 1.1–2.1 times as broad as long; anterior margin moderately concave in adult males, straight to slightly convex beside and just forward of eyes; pectoral fin apices broadly rounded; posterior margin slightly convex; free rear tip broadly rounded. Head length long 16.7–24.3% TL; preorbital snout length long 9.7–16.9% TL; preoral length relatively long 11.8–17.5% TL. Snout tip broadly triangular, possessing no fleshy process at apex. Deeply concave space directly behind eyes, eye length relatively small 2.6–5.0% TL. Interorbital width short 3.3–5.3% TL. Spiracles average 1.6–3.7% TL, oval shaped. Mouth width short 6.2–10.0% TL. Nasal curtain length average 2.8–4.5% TL, width average 7.0–9.0% TL, its posterior margin fringed at the corners; anterior margin of curtain lobe-like. Internarial distance 6.6–7.7% TL; first gill slit length 1.2–2.5% TL; fifth gill slit length 0.7–2.1% TL; distance between first gill slits large 14.7–20.2% TL, and distance between fifth gill slits 10.1–13.4% TL. Upper jaw moderately well arched, possessing a symphysis; lower jaw convex. Teeth similar in both jaws; teeth unicuspid, with a strong, bluntly pointed posteriorly directed cusp; arranged in longitudinal rows; upper and lower teeth high in number (34–42 and 32–38, respectively).
Pelvic fins average overall; anterior lobe short 6.9–9.9% TL, posterior lobe relatively long 7.2–15.7% TL, and similar between sexes and maturities, inner margin deeply incised. Tail relatively long 51.1–60.9% TL, rather slender, wider at base, tapering to the first dorsal fin origin, not expanded in the middle. Lateral tail fold length 13.9–24.0% TL, similar in both sexes, not obviously broader at any point along its length. Dorsal fins moderate in size and shape, both dorsal fins similar in height, 1.9–3.0% TL and 1.7–3.1% TL, respectively; bases of both dorsal fins are the same length, 2.8–5.0% TL; anterior margins of both fins concave, apices rounded; free rear tip rounded, occasionally pointed; interdorsal space large 1.3–3.1% TL, with larger individuals having a shorter interdorsal space, rear tip of first dorsal fin not overlapping base of second dorsal fin. Caudal fin moderate, low, height 0.5–2.1% TL; its dorsal margin weakly concave; connected to second dorsal fin by a small membranous ridge.
Dorsal surface covered with fine prickles, with larger prickles on the tail; ventral surface smooth. Scapular, middorsal, nuchal, interdorsal, and tail thorns present, males with a well-developed set of alar thorns; malar thorns absent; thorns vary slightly in size, from moderate to well-developed. Middorsal thorns well-developed and high in number (4–11); nuchal thorns very well-developed and high in numbers (3–5); 1–2 scapular thorns present on either side; tail thorns average (25–30); interdorsal thorns weakly developed (1–2). Alar thorn patches range between 7–8 rows and 21–24 columns on both pectoral fins. No multiple rows of thorns on body.
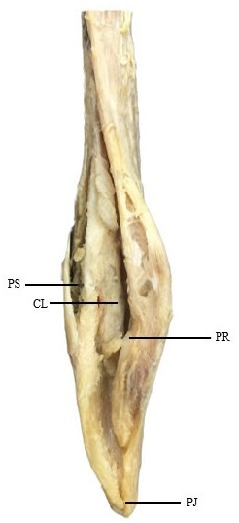
Mature claspers relatively short and stubby, base length 1.3% TL, inner length 23.1% TL, and tip of clasper rounded and not bulbous ( Figure 8 View FIGURE 8 ). Clasper inner length 42.6% of tail length; weakly defined pseudosiphon present, its length 30.8% of the clasper length; inner surface of dorsal lobe possesses a short pseudorhipidion; U-shaped cleft; inner surface of ventral lobe has a rounded projection.
Clasper skeleton consists of 3 dorsal terminal, 1 accessory terminal, ventral terminal and axial cartilages; dorsal terminal 1 large, taking up most of the dorsal surface; dorsal terminal 1 forms a weakly defined pseudosiphon externally; the tip of the dorsal marginal is round, and forms a thick, short, but poorly defined pseudorhipidion externally; ventral terminal curved and pointed, possessing a tip that forms the projection externally; accessory terminal 1 almost as long as the ventral terminal; tip of accessory terminal 1 is pointed.
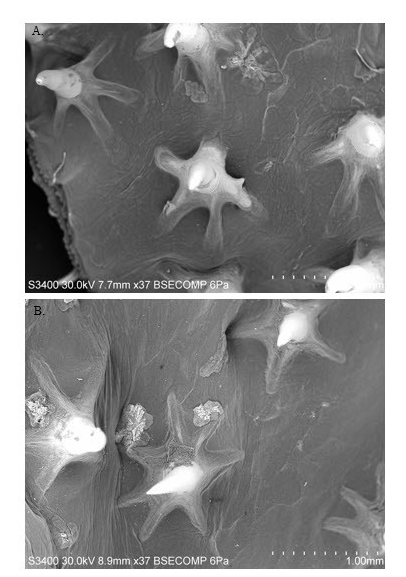
Dermal denticles possess 4–7 points at the base; developed on the posterior third of the dorsal surface; denticles on the first dorsal fin strong curved, claw-like; denticles on head wider than dorsal fin; found in high density patches ( Figure 9 View FIGURE 9 ).
Length of rostral cartilage 47.2% of cranial length; prefontanelle rostral length 48.4%; cranial width 87.3% least interorbital width 18.3%; length of anterior prefontanelle 11.1%; length of posterior prefontanelle 11.1%; length of rostral appendices 20.6%. Rostral cartilage nearly straight; the two fontanelles are equal in length ( Figure 10 View FIGURE 10 ).
Coloration. Dorsal coloration dark brown to grey, with dark spots on pectoral fins, often darker at the disc margins. One ocellus on either pectoral fin is present in some specimens. Spiracles often pale in coloration. Ventral coloration white, including the claspers, but with dark brown to dark grey coloration on the snout, gills, posterior disc margins, pelvic fins, cloaca, and underside of the tail. Thorns on dorsal surface pale. Coloration after preservation is brown dorsally and pale ventrally, with the dark spots on pectoral fins largely becoming lighter and closer in color to the rest of the surface.
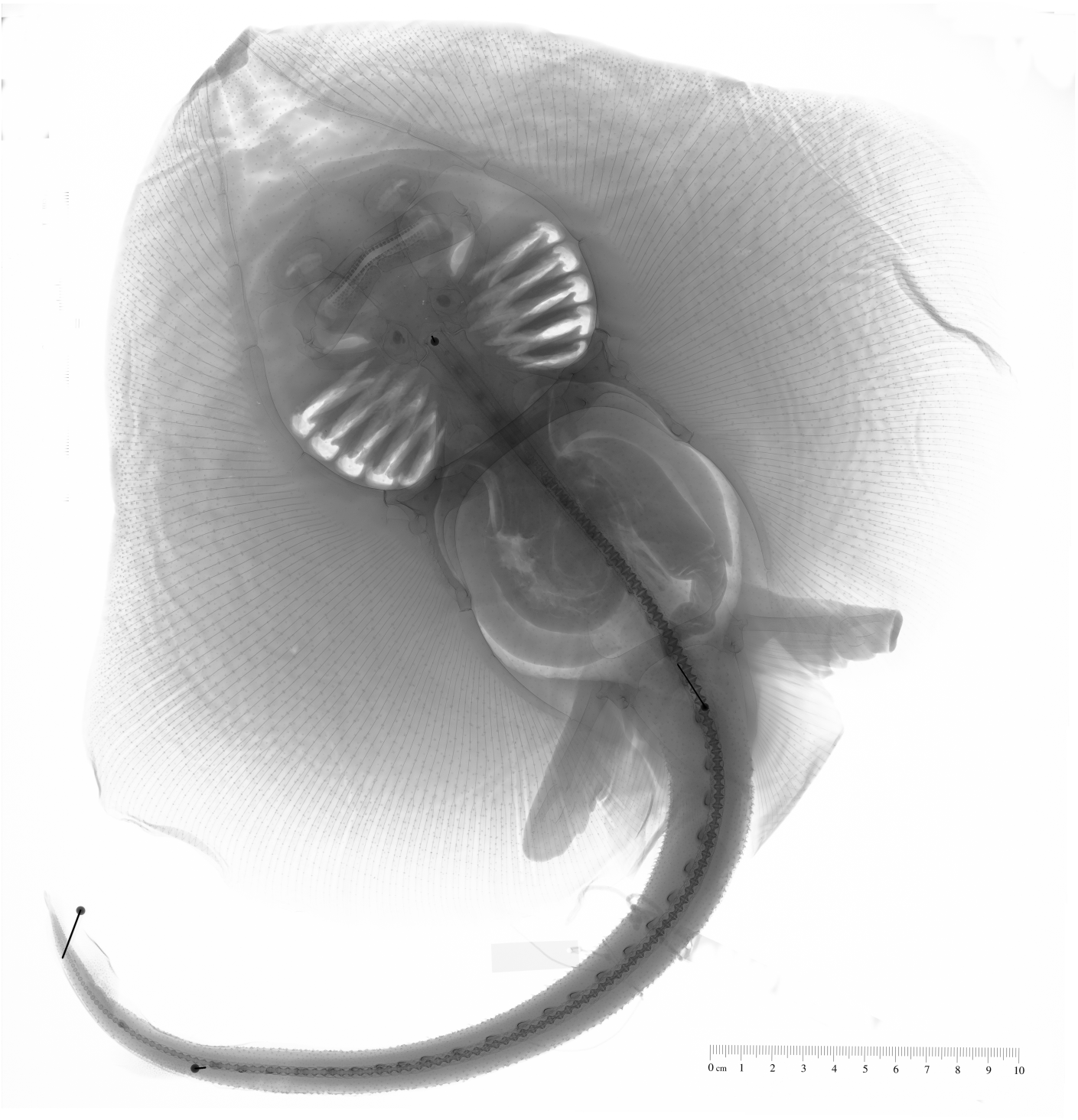
Egg case description. Egg cases large (> 120 mm TL), golden brown in color, and covered by coarse, striated, and anteriorly-directed prickles, which give is a velvety texture. Cases possess long, curved horns at each corner, with the anterior horns being short. Horns taper and become thin and filamentous at their ends ( Ebert & Davis, 2007).
Distribution. Bathyraja aleutica has been confirmed as occurring in the North Pacific, specifically from the Bering Sea, south to Cape Mendocino, northern California, and as far west as northern Japan ( Ebert, 2003). It occurs at depths of 15–1,602 m ( Kyne et al., 2012); the specimens included in this study were all from the shallow portion of the species’ depth range ( 379–730 m).
Biological notes. Size at maturity for males 113 cm TL; 125 cm TL for females. Males grow to at least 150 cm TL; females grow to about 154 cm TL. Size at birth is 12–15 cm TL ( Ebert, 2003, 2005). Maximum size is at least 154 cm TL ( Haas, 2011; Haas et al., 2016). Primarily consumes benthic crustaceans, including shrimps and crabs, and secondarily feeds on bony fishes ( Brown et al., 2011).
Habitat. Inhabits deep waters, on the outer continental shelf and upper slope, often over muddy sediment ( Ebert, 2003).
Etymology. The species was named after the Aleutian Islands, where the holotype was collected.
Comparisons. Bathyraja aleutica is a relatively large skate that can be easily separated from other smallerbodied skates in its range (e.g., B. interrupta , B. kincaidii , B. microtrachys , and B. trachura ) based on coloration, thorn counts, and morphological measurements. Of the small skates, only B. microtrachys possesses similar dorsal and ventral coloration, but lacks middorsal, nuchal, and scapular thorns. Interdorsal space is significantly larger than all of its congeners (F 6,104 = 15.3, p <0.0001). Furthermore, middorsal thorns are significantly higher in number than all of its congeners (F 6,104 = 16.2, p <0.0001).
Bathyraja spinosissima and B. abyssicola are similar in size and range to B. aleutica , but the pale coloration and lack of middorsal, nuchal, and scapular thorns in the former easily separates it. Bathyraja abyssicola can be distinguished from B. aleutica in that it possesses 0–2 middorsal thorns and B. aleutica has 4–11 in the same region. Mature claspers can also be used, as B. abyssicola is characterized as having long, thin claspers with strong, Vshaped clefts, compared to the short, stubby claspers with weak U-shaped clefts in B. aleutica .
Remarks. Nursery regions have been identified on the continental shelf-slope interface in the southeastern Bering Sea ( Hoff, 2007, 2010). Eggs are deposited on substrate from June to November ( Hoff, 2002; Ebert, 2003). This species has been reported to exhibit complete albinism, with an albino specimen coming from Prince William Sound ( Bigman et al., 2015).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bathyraja aleutica ( Gilbert, 1896 )
| Knuckey, James D. S. & Ebert, David A. 2022 |
Bathyraja aleutica :
| Dyldin, Y. V. & Orlov, A. M. 2021: 58 |
| Burton, E. J. & Lea, R. N. 2019: 32 |
| Dyldin, Y. V. & Orlov, A. M. 2018: 168 |
| Ebert, D. A. & Bigman, J. S. & Lawson, J. M. 2017: 21 |
| Weigmann, S. 2016: 90 |
| Kells, V. & Rocha, L. A. & Allen, L. G. 2016: 78 |
| Dyldin, Y. V. 2015: 61 |
| Shinohara, G. & Nakae, M. & Ueda, Y. & Kojima, S. & Matsuura, K. 2014: 234 |
| Parin, N. V. & Evseenko, S. A. & Vasil'eva, E. D. 2014: 30 |
| Page, L. M. & Espinosa-Perez, H. & Findley, L. D. & Gilbert, C. R. & Lea, R. N. & Mandrak, N. E. & Mayden R. L. & Nelson, J. S. 2013: 55 |
| Ebert, D. A. & Compagno, L. J. V. 2007: 116 |
| Ebert, D. A. & Davis, C. D. 2007: 4 |
| Love, M. S. & Mecklenburg, C. W. & Mecklenburg, T. A. & Thorsteinson, L. K. 2005: 11 |
| Nelson, J. S. & Crossman, E. J. & Espinosa Perez, H. & Findley, L. T. & Gilbert, C. R. & Lea, R. N. & Williams, J. D. 2004: 55 |
| Ebert, D. A. 2003: 195 |
| Orlov, A. M. 2003: 45 |
| Fedorov, V. V. & Chereshnev, I. A. & Nazarkin, M. V. & Shestakov A. V. & Volobuev, V. V. 2003: 15 |
| Hoff, G. R. 2002: 145 |
| Sheiko, B. A. & Fedorov, V. V. 2000: 15 |
| Dolganov, V. N. 1999: 429 |
| Compagno, L. J. V. 1999: 488 |
| McEachran, J. D. & Dunn, K. A. 1998: 286 |
| Stehmann, M. F. W. 1986: 263 |
| Masuda, H. & Amaoka, K. & Araga, C. & Uyeno, T. & Yoshino, T. 1984: 14 |
| Eschmeyer, W. N. & Herald, E. S. & Hammann, H. 1983: 49 |
Breviraja aleutica :
| Ishiyama, R. 1952: 9 |
Raja aleutica :
| Gilbert, C. H. 1896: 397 |