Spinomantis beckei, Glaw, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4317.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:60Ebaa17-D222-47F8-8549-E7E62F7C4926 |
|
DOI |
https://doi.org/10.5281/zenodo.6036040 |
|
persistent identifier |
https://treatment.plazi.org/id/2E9DE45A-334E-435D-A2AF-9207900C9E49 |
|
taxon LSID |
lsid:zoobank.org:act:2E9DE45A-334E-435D-A2AF-9207900C9E49 |
|
treatment provided by |
Plazi |
|
scientific name |
Spinomantis beckei |
| status |
sp. nov. |
Spinomantis beckei View in CoL sp. nov.
Remark. This species has previously been referred to as Spinomantis bertini by Vences et al. (2006), Glaw & Vences (2007), Vieites et al. (2009) and Perl et al. (2014). Note that sequences assigned to candidate species Spinomantis sp. Ca 6 in Vieites et al. (2009) and Perl et al. (2014) are now assigned to S. bertini .
Holotype. ZSM 173 View Materials /2005 ( FGZC 2431 ), adult male ( Figs. 2 View FIGURE 2 A,B and 3D̄F), collected along a stream at high elevation in Andohahela National Park (coordinates not taken but at ca. 1–3 km south from campsite at S24.54403, E46.71412, and at approximately 1650 m above sea level), Toliara Province, southeastern Madagascar, on 21 January 2005, by F. Glaw, M. Vences, and P. Bora. GoogleMaps
Paratype. ZSM 174/2005 (FGZC 2488), adult male ( Fig. 2 View FIGURE 2 C,D), with same collecting data as holotype.
Diagnosis and comparisons. Assigned to the genus Spinomantis within the Mantellidae mainly based on phylogenetic affinities to S. bertini , and supported by (1) presence of intercalary elements between ultimate and penultimate phalanges of fingers and toes (verified by external shape of digits), (2) presence of distinct femoral glands of type 2 ( Glaw et al. 2000) in males, (3) paired to bilobed subgular vocal sacs, (4) outer metatarsal tubercle present, (5) maxillary teeth present, and (6) adults living along streams in rainforest.
Within Spinomantis View in CoL , according to comparative data from Glaw & Vences (2007) and Cramer et al. (2008), the new species is distinguished from the fully arboreal species S. aglavei View in CoL , S. fimbriatus View in CoL , S. massi View in CoL , S. nussbaumi View in CoL , S. peraccae View in CoL , S. phantasticus View in CoL , and S. tavaratra View in CoL by terrestrial to scansorial habits, a much smaller body size (SVL 22̄25 vs. 31̄ 56 mm), and from S. aglavei View in CoL , S. fimbriatus View in CoL , S. massi View in CoL , S. phantasticus View in CoL , and S. tavaratra View in CoL by a smooth skin without dermal spines and flaps; from S. elegans View in CoL by a much smaller size (SVL 22̄25 vs. 50̄ 60 mm) and a different colour pattern; from S. guibei View in CoL by smaller size (SVL 22̄25 vs. 29̄ 40 mm) and a colour pattern without distinct green elements; from S. microtis View in CoL by smaller size (SVL 22̄25 vs. 30̄ 48 mm), less enlarged terminal discs on fingers and toes, smooth (vs. shagreened) skin, and different colour pattern; and from S. brunae View in CoL by smaller size (SVL 22̄25 vs. 32̄ 35 mm) and a different colour pattern. Furthermore, the new species differs from the other species and candidate species of the Spinomantis bertini View in CoL complex ( Table 1) by a substantial genetic differentiation with pairwise divergences of 5.5̄10% in the mitochondrial 16S rRNA gene and the analysed specimens of S. beckei View in CoL form a monophyletic group based on this mitochondrial marker ( Fig. 1 View FIGURE 1 ).
According to molecular data ( Fig. 1 View FIGURE 1 ) Spinomantis beckei View in CoL is the sister species of S. bertini View in CoL , which also is morphologically the most similar representative of the genus. Furthermore, both species occur in the Andohahela Massif though at different elevations. The new species differs from S. bertini View in CoL by details of coloration, especially the more brown dorsal colour (vs. olive-greenish shade in S. bertini View in CoL , with greenish elements especially on the dorsal surfaces of the limbs; Fig. 4 View FIGURE 4 ), less contrasted crossbands on shanks, thighs and lower arms, and a less expressed frenal stripe which runs as a thin white line from behind the insertion of forelimbs to eyes and fades anterior to eyes, while it is broader, with greenish shade, and running from behind the forelimb insertion to snout tip in S. bertini View in CoL ( Fig. 4 View FIGURE 4 ). The new species probably also differs from S. bertini View in CoL by a faster note repetition rate in advertisement calls (see Discussion). It furthermore differs from S. bertini View in CoL by a substantial genetic differentiation of 5.5̄6.3% in the mitochondrial 16S rRNA gene ( Table 1), and the two species are reciprocally monophyletic in the phylogenetic tree based on 16S sequences ( Fig. 1 View FIGURE 1 ). Perl et al. (2014) provided DNA barcoding data for S. beckei View in CoL (holotype ZSM 173/2005, GenBank accession number JN133263 View Materials ; as S. bertini View in CoL ) and S. bertini View in CoL (FGZC 148, JN133264 View Materials ; as S. sp. Ca6), and the two species had a pairwise uncorrected divergence of 12.2% for the sequenced fragment of the cytochrome oxidase I (COI) gene.
Description of the holotype. Specimen slightly dehydrated, but in reasonable state of preservation, muscle tissue sample removed from right side of thigh. Snout̄vent length 24.5 mm, for further measurements see Table 2. Body slender; head longer than wide, slightly wider than body; snout rounded in dorsal and lateral views, nostrils directed laterally, slightly protuberant, nearer to tip of snout than to eye; canthus rostralis distinct, straight; loreal region slightly concave; tympanum distinct, rounded, its diameter 78% of eye diameter; supratympanic fold indistinct; tongue small, ovoid, bifid and free posteriorly; vomerine teeth absent, maxillary teeth poorly recognizable; choanae small, rounded. Arms slender, subarticular tubercles single; fingers without webbing; relative length of fingers 1<2<4<3; finger disks distinctly enlarged; nuptial pads absent. Hind limbs slender; tibiotarsal articulation reaches beyond eye when the hind limb is adpressed along the body; lateral metatarsalia partly connected; inner metatarsal tubercles distinct, outer metatarsal tubercle not recognizable; webbing formula between toes rudimentary, 1(1), 2i (1.5), 2e(1), 3i (2.5), 3e(2), 4i (3), 4e(3), 5(2); relative length of toes 1<2<3=5<4. Skin on the upper surface smooth, without folds or ridges. No distinct enlarged tubercles in the cloacal region; ventral skin smooth. Femoral glands large and distinctly elevated, measuring 5.9 x 2.7 mm, of type 2 sensu Glaw et al. (2000), distance between femoral glands 0.9 mm.
After eleven years in preservative, the dorsum appears rather uniformly dark greyish-brown. There is a moderately distinct dark stripe between the lighter flanks and the lighter dorsum, running below the dorsolateral border from the posterior eye to the inguinal region. A further dark band between the nostril and the anterior border of the eye. The hind limbs are dorsally brown with indistinct dark brown crossbands and light mottling on the thigh and shank; tarsus brown. Arms and hands dorsally largely uniformly brown. Tympanum brown. A thin white line is running along the upper lip, starting below the eye, posteriorly encircling the the insertion of forelimbs (frenal stripe). Ventrally, blackish with numerous white dots and small markings on chest and venter. Throat largely uniformly brown, except two light lateral patches, probably corresponding to the vocal sacs, which presumably are paired or bilobate subgular. Ventral side of arms with brown and beige spots. Ventral sides of thighs, shanks, and feet brown with contrasting white spots. Femoral glands largely brown with few light spots. Ventral sides of hands and feet uniformly brown.
Coloration in life ( Fig. 2 View FIGURE 2 A,B) similar to that in preservative, but generally lighter and more contrasting. Ground colour of ventral side black, femoral glands brown marbled with beige.
Variation. The paratype specimen ( Fig. 2 View FIGURE 2 C,D) is a slightly smaller adult male of 22.2 mm SVL (see Table 2 for additional measurements). Compared to the holotype, the femoral glands are less distinct and smaller ( Table 2). The ventral side has a more extensive light patterning, with broader whitish vermiculation in life. In life, dorsally some weak greenish shade recognizable on shanks, but generally the dorsal colour is dominated by different tones of brown.
Advertisement call. Advertisement calls of Spinomantis beckei were recorded on 28 January 2005 at Andohahela National Park at approximately 1650 m a.s.l. at an air temperature between 15–20°C (seven calls with a total of 57 notes of one individual recorded). The recorded calls were emitted either by the holotype or the paratype (insufficient field notes taken) at apparently regular motivation. Specimens were calling in the late afternoon before dusk, during a period of very heavy rains caused by a cyclone passing. This recording was incorrectly assigned to S. bertini by Vences et al. (2006: CD2, track 57).
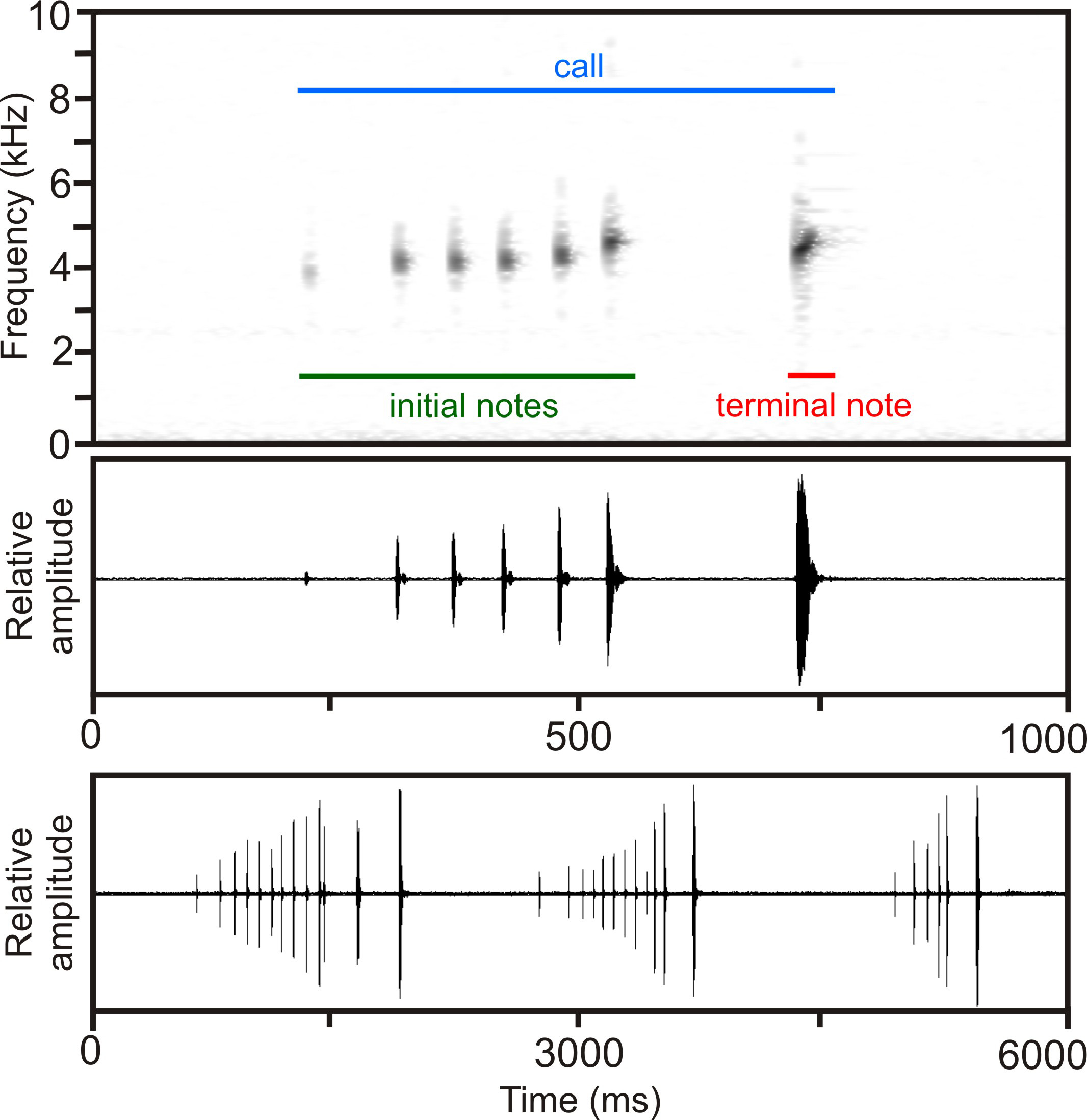
Calls ( Fig. 5 View FIGURE 5 ) have a duration of 430–1278 ms (696 + 313 ms) and consist of a series of 6–13 (8.5 + 3.1) short notes, with each note represented by one single pulse. Calls are somewhat complex in structure due to different kinds of modulation involved. Initial notes (i.e., those excluding the terminal note[s] with maximum amplitude) are very short with a duration of 5–13 ms (8.4 + 2.9 ms) and are emitted at intervals of 50–89 ms (63 + 11 ms) duration at an approximate repetition rate of 13–14 notes/second. Initial notes are followed by 1–2 terminal notes of longer duration of 20–26 ms (23.6 + 1.9 ms), which are separated from initial notes by longer inter-note intervals of 163– 195 ms (172 + 11 ms), but are generally identical to initial notes in overall structure. Terminal notes exhibit the highest amplitude of all notes of a call, with the amplitude almost constantly increasing in series of short initial notes. However, the increase in amplitude from the first to the last note is slightly irregular in some calls. In addition to the amplitude modulation present within calls, there is also slight frequency modulation among notes, with the first note exhibiting the lowest dominant frequency with 4016–4317 Hz (4095 + 124 Hz), increasing towards the last of the short initial notes with 4468–4790 Hz (4670 + 133 Hz). The longer and more powerful terminal note of each call then slightly drops in dominant frequency with 4297–4467 Hz (4379 + 54 Hz), with this frequency representing the overall dominant frequency of the entire call. The approximate prevalent bandwidth of the call is 3400–6700 Hz. Calls were emitted at an approximate rate of 30 calls/minute, with inter-call intervals ranging from 840–1690 ms. The combination of different note lengths, amplitude modulation and frequency modulation results in a very particular and easily recognizable sound of the call.
In comparison, calls from the type locality of S. bertini recorded at 21°C air temperature at Isaka-Ivondro, Andohahela, described and illustrated by Andreone & Randriamahazo (1997), differ by the lack of longer terminal note(s), distinctly lower note repetition rate (7.3 versus 13–14 notes/second) and absence of or less distinct frequency modulation among notes within a call. However, note duration and bandwidth seem to overlap (no recording available to us for detailed comparison).
Calls of an undescribed candidate species of the Spinomantis bertini complex (S. sp. Ca7; Vieites et al. 2009; Perl et al. 2014) from Mount Maharira, Ranomafana National Park (Vences et al. 2006: CD2, track 58, as Spinomantis aff. bertini ), mainly differ from calls of S. beckei by a lower number of notes per call (3–4 versus 6– 13), shorter call duration (293–400 versus 430–1278 ms), notes consisting of several pulses (versus single pulse only), and a slight decrease of frequency of notes within a call (versus distinct increase of frequency).
Natural history and distribution. Spinomantis beckei was found in humid montane forest along a small rainforest stream in the rainy season where the male frogs were calling in the afternoon from the ground, hidden in low riparian vegetation and small cavities. The species is only known from high elevations of the Andohahela National Park (inset map in Fig. 1 View FIGURE 1 ).
Etymology. The new species is named after Konrad Becke in recognition of supporting biodiversity research and nature conservation through the BIOPAT initiative.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Spinomantis beckei
| Glaw, Frank 2017 |
S. beckei
| Glaw 2017 |
S. beckei
| Glaw 2017 |
Spinomantis beckei
| Glaw 2017 |
Spinomantis beckei
| Glaw 2017 |
S. beckei
| Glaw 2017 |
Spinomantis
| Dubois 1992 |