Austrocnephia strenua ( Mackerras & Mackerras 1950 ), 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4627.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A7802D6F-D366-44DE-82D9-F0AAC7468157 |
|
persistent identifier |
https://treatment.plazi.org/id/038A87CC-FF81-4422-FF30-F8DAAA9F571E |
|
treatment provided by |
Plazi |
|
scientific name |
Austrocnephia strenua ( Mackerras & Mackerras 1950 ) |
| status |
|
Austrocnephia strenua ( Mackerras & Mackerras 1950) View in CoL . New combination.
( Figs. 58 View FIGURES 58–61 –98)
Cnephia strenua Mackerras & Mackerras 1950: 170 View in CoL ; original description. Mackerras & Mackerras, 1955: 105.
Stegopterna (Z) strenua View in CoL . Colbo, 1974: 67; unpublished reassignment.
Cnephia strenua View in CoL . Rothfels, 1979: 522.
‘ Cnephia View in CoL of authors’ strenua . Crosskey, 1987: 443; Prosimuliini , undetermined genus.
strenua View in CoL . Crosskey, 1989: 222; unplaced species of Prosimulinii.
Paracnephia strenua . Crosskey & Howard, 1997: 18; Prosimuliini , new combination
‘ Cnephia ’ strenua View in CoL . Moulton, 2000: 110. Moulton, 2003: 47.
Paracnephia strenua. Crosskey & Howard, 2004: 10 ; Prosimuliini View in CoL , unplaced to subgenus.
Paracnephia strenua. Adler & Crosskey, 2008: 26 , transferred to Simuliini View in CoL . Adler, 2019:32; unplaced to subgenus.
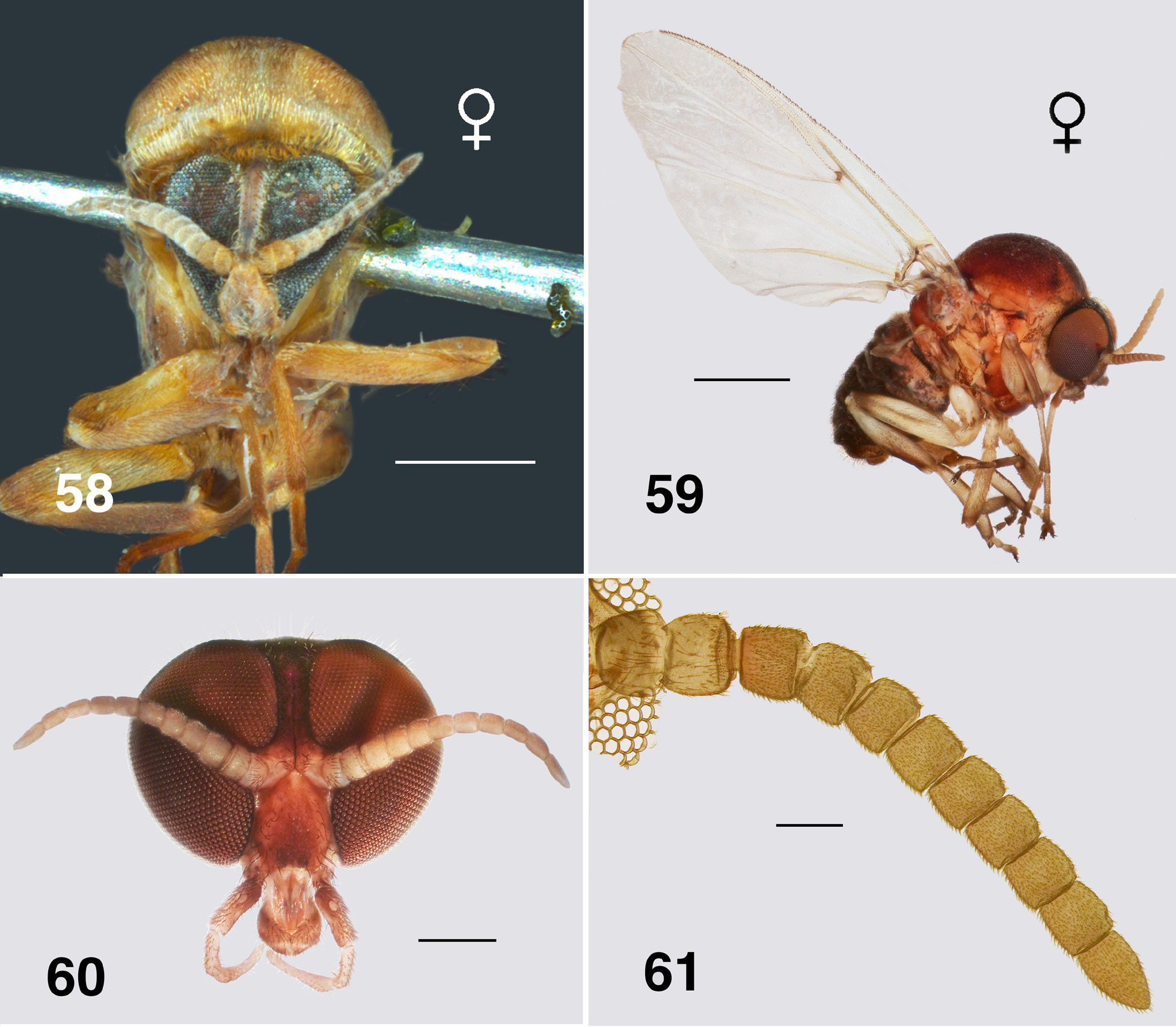
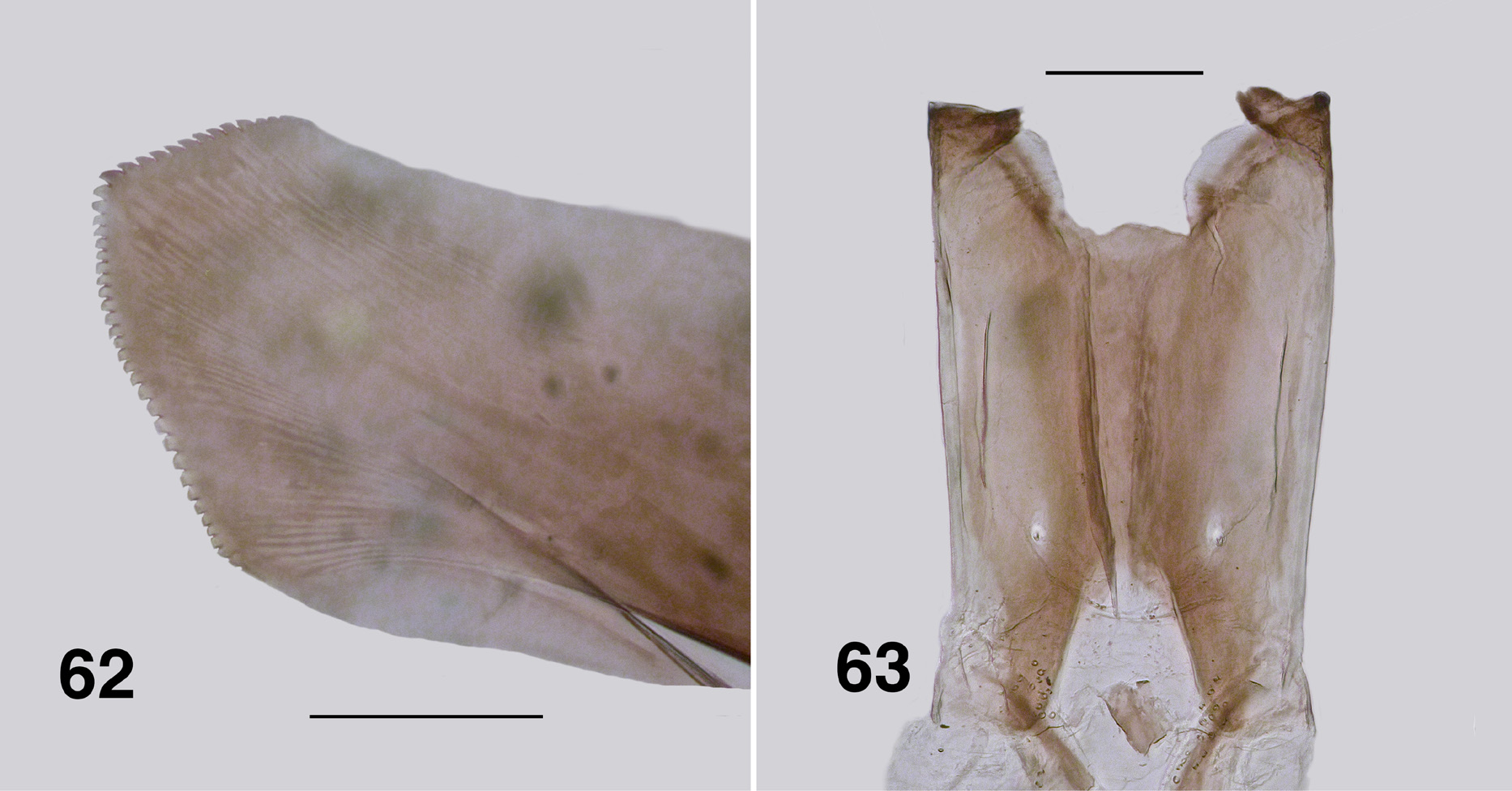
Redescription. Adult female (based on large numbers of adults in alcohol). Body ( Figs. 58, 59 View FIGURES 58–61 ): head brown, thorax dark orange to dark yellow, abdomen overall black, yellowish anteriorly; total length 2.8–3.3 mm. Head ( Fig. 60 View FIGURES 58–61 ): width 0.97–1.00 mm; depth 0.70–0.73 mm; postocciput with dense yellow vestiture, frons narrow, parallel-sided, dark brown-black, vestiture of sparse hairs; frons:head width ratio 1.0:10.7; postocciput dark, vestiture of dense hairs. Eyes: interocular distance 0.09–0.11 mm; ommatidia diameter 0.025 mm; ca. 36 rows across and 48 down at mid-eye. Clypeus: width 0.25 mm; mottled in colour. Antenna ( Fig. 61 View FIGURES 58–61 ): elongate, extended well beyond posterior margin of head; total length 1.1–1.4 mm; scape and pedicel pale and similar in size to darker flagellomere I; flagellomeres II–VII, similar in size, brown, tapered finely to elongated apical flagellomere IX. Mouthparts: well developed, ca. 0.30–0.41× head depth; maxillary palp length 0.8–1.1 mm, palpomeres I & II markedly small, palpomere III darker brown than remainder, not densely hirsute, palpomere V twice as long as palpomere IV, proportional lengths of palpomeres III–V 1.0:0.6:1.3; sensory organ elongated, 0.5× length of palpomere III, opening large, 0.5× vesicle length; mandible ( Fig. 62 View FIGURES 62, 63 ) of Souita Fall population is unique with ca. 14 outer teeth, 29–32 inner teeth, ca.
12 of those recurved and sharp, remainder angulate and blunt, other populations possess just a few outer teeth, most only inner teeth; lacinia with 19–21 outer and 9–13 inner teeth, smaller than in aurantiaca ; cibarium ( Fig. 63 View FIGURES 62, 63 ) with medial depression angulate and with apex convex, cornuae broad basally and markedly flared, slightly sculpted. Thorax: length 1.7–2.0 mm; width 1.3–1.7 mm; overall dark yellowish orange, with distinct dorsocentral vittae of golden scales and pale lateral lines; postpronotal lobe well developed with vestiture similar to scutum—even sparse fine small hairs; scutellum markedly paler than scutum, vestiture of sparse very fine yellowish hairs centrally, distinct longer black hairs laterally; postnotum concolourous with scutellum, vestiture similar; antepronotal lobe with sparse yellow hairs; proepisternum and fore coxa with sparse hairs; anepisternal membrane yellowish brown, bare; katepisternum dark brown, as long as deep, sulcus shallow and broad. Wing ( Fig. 64 View FIGURE 64 ): length 4.2 mm; width 2.0 mm; membrane occasionally very slightly fumose on apex and anal lobe, with lightly pigmented spot at junction of r-m cross vein and R 1; distal 2/3 of costa with mixture of hairs and spines, Rs narrowly divided distally (R 2+ 3 in some specimens expressed as simple row of hairs); area between Sc and R light yellow; a:b ratio 1.0:2.5; basal medial cell minute or absent; M 1 typically appearing doubled, sometimes tripled; CuA not markedly sinuous; CuP extended to wing margin in some specimens; A 1 not reaching margin. Haltere: stem clear, knob dark tan. Legs: coxae and femora mostly yellowish, the latter dark brown apically; tibiae with basal quarter yellowish, remainder dark; hind basitarsus with ventral row of sparse stout spines; calcipala markedly longer than wide ( Fig. 65 View FIGURES 65–68 ); pedisulcus not markedly developed—at most represented by wrinkled cuticle; tarsomere II 2.5–3.1× longer than apical width; claw ( Fig. 66 View FIGURES 65–68 ), with main talon strongly curved and evenly tapered, slightly serrated along inner edge, basal tooth 0.3× length of claw, heel rounded. Abdomen: basal scale medium brown, vestiture of long dense hairs; anterior few segments yellowish, remaining posterior segments mottled blackish brown; tergite II weakly sclerotized and V-shaped, tergites III & IV rectangular, broader in more posterior segments; vestiture markedly expressed in segments III and further back. Genitalia: markedly small; sternite VIII with distinct microtrichial array medially, larger stronger hairs posterolaterally; hypogynial valves ( Fig. 67 View FIGURES 65–68 ), lightly pigmented, vestiture of triads of microtrichia and strong hairs, medial edges of valves slightly convex but not touching, anteromedial edges slightly strengthened; narrowly rounded apically with poorly defined edges, medially with raised area; genital fork ( Fig. 68 View FIGURES 65–68 ) with anterior stem relatively long and narrowed, deflected slightly ventrally, slightly expanded apically, indication of membranous lateral area in stained specimens, posterolateral arms narrow, lateral plate trapezoid or diamond-shaped, without anteriorly directed apodeme; spermatheca ovoid ( Fig. 70 View FIGURES 69–71 ), elongated, dark brown, lightly tuberculate, internal fine spines (acanthae) absent, with small rounded membranous area at junction with spermathecal duct; cercus ( Fig. 69 View FIGURES 69–71 ) in lateral view bluntly cone-shaped, slightly constricted medially; anal lobe subequal in size to cercus, both with vestiture of long hairs and dense microtrichia. Egg: sub-triangular in lateral view ( Fig. 71 View FIGURES 69–71 ), ca. 0.19 by 0.11 mm, with ca. 600 counted in a single dissected female from Lamington National Park, Queensland.
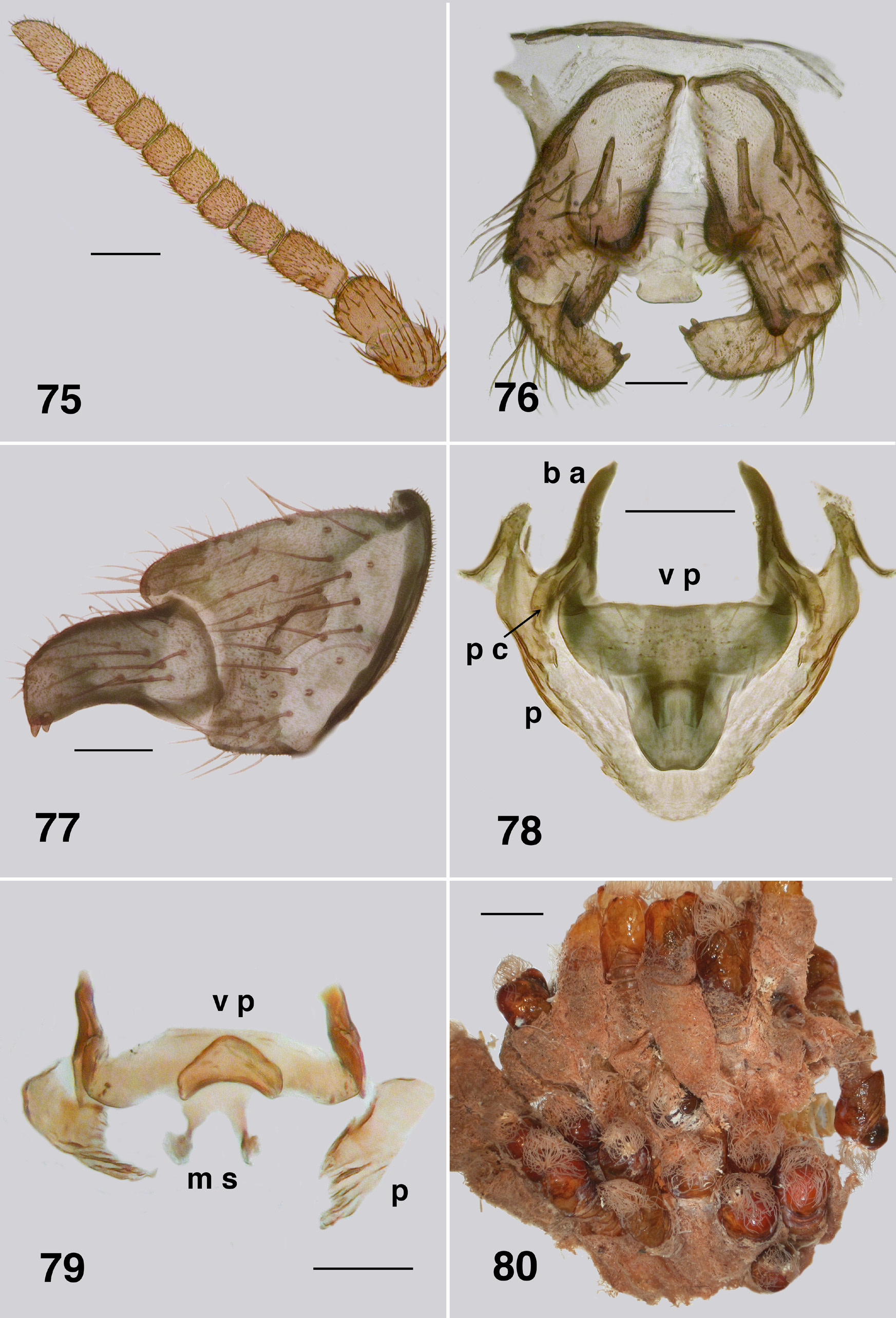
Adult male (numerous specimens). Body: pinned specimens light brownish orange (Fig. 72), dark brownish orange and black in ethanol preserved specimens (Fig. 73); total length 3.3–3.5 mm. Head (Fig. 74): width 1.0– 1.2 mm; depth 0.87–1.0 mm. Eyes: upper ommatidia dark brown, enlarged, diameter 0.076 mm, ca. 13–16 across and down; lower ommatidia black to blackish orange/brown, markedly smaller, diameter 0.027 mm, 34–36 across and down. Clypeus: blackish brown; width 0.19–0.27 mm; vestiture of sparse fine black hairs. Antenna ( Fig. 75 View FIGURES 75–79 ): markedly elongated, total length 0.76–1.0 mm; pedicel and scape darker; scape and pedicel subequal in size, flagellomere I narrower than scape and twice as long as wide, pale basally, flagellomeres less tapered than those in A. aurantiaca , overall light brown. Mouthparts: insubstantial; length 0.14–0.20× head depth; maxillary palp, essentially as for A. aurantiaca , 0.55–0.92 mm long, palpomeres I & II small, palpomere III shorter than palpomere IV, palpomere V elongated, proportional lengths of palpomeres III–V 1.0:0.6:1.6, sensory vesicle small, occupying 0.33× palpomere width, opening 0.5× vesicle width; lacinia small, lacking teeth, but with apical hairs; mandible weakly developed and lacking teeth. Thorax: markedly domed, head angled anteriorly; length 1.6 mm; width 1.3 mm; postpronotal lobe with longish fine pale hairs, concolourous with scutum; antepronotal lobe with distinct patch of fine pale hairs, proepisternum bare; scutum evenly yellow, vestiture of evenly sparse short fine pale hairs, dense and long in scutellar depression; scutellum lighter than scutum, with markedly elongate yellow and black hairs; pleuron brown, anepisternal membrane bare, katepisternum dark brown, sulcus distinct, but shallow; metathoracic furcasternum lacking flared anterior flanges. Wing: length 3.6–3.9 mm, width 1.9–2.0 mm; a:b ratio 1.0:2.6; otherwise as for female. Haltere: stem pale, knob tan. Legs: overall yellowish, lighter than female; calcipala markedly longer than wide; pedisulcus poorly developed, at most represented by wrinkled cuticle; tarsal claw with truncated basal tooth and grappling hook of 23–26 teeth. Abdomen: black dorsally and posteriorly, pale anteroventrally, basal scale black, hairs black, extended to posterior of segment IV on contracted abdomen, tergites poorly sclerotized, tergite II 2.5× as wide as long, pigmented only medially, tergite III, narrower, but pigmented laterally, other tergites broader, increasingly so posteriorly and less fully pigmented, vestiture of sparse long hairs, more so posteriorly; sternites moderately developed and hirsute, more so posteriorly. Genitalia: small, not heavily pigmented ( Fig. 76 View FIGURES 75–79 ); cerci well developed; gonocoxa 1.3× longer than basal width, posteromedially strengthened and slightly fluted, margin extended beyond articulation with gonostylus, hirsute with long black sparse hairs and microtrichia; gonostylus in ventral view narrowed, approximately 1.8× longer than basal width, with two substantial apical spines ( Fig. 77 View FIGURES 75–79 ); ventral plate small, 1.7× wider than long in ventral view with variably-shaped posterior lip; Souita Falls population ( Fig. 76 View FIGURES 75–79 ) with lip short, narrowed at base and with broadly rounded apex; Behana Gorge population ( Fig. 78 View FIGURES 75–79 ) with lip long, not narrowed at based, and with apex narrowly rounded); basal arms elongated; paramere connector short and broad; median sclerite with two short variably expressed sclerotized arms projected dorsally ( Fig. 79 View FIGURES 75–79 ); paramere in all populations triangular, plate-like basally, slightly strengthened along ventral edge, tapered distally with irregu- lar ridges; adeagal membrane either bare or with a few, short, weakly expressed spinules near apex of paramere.
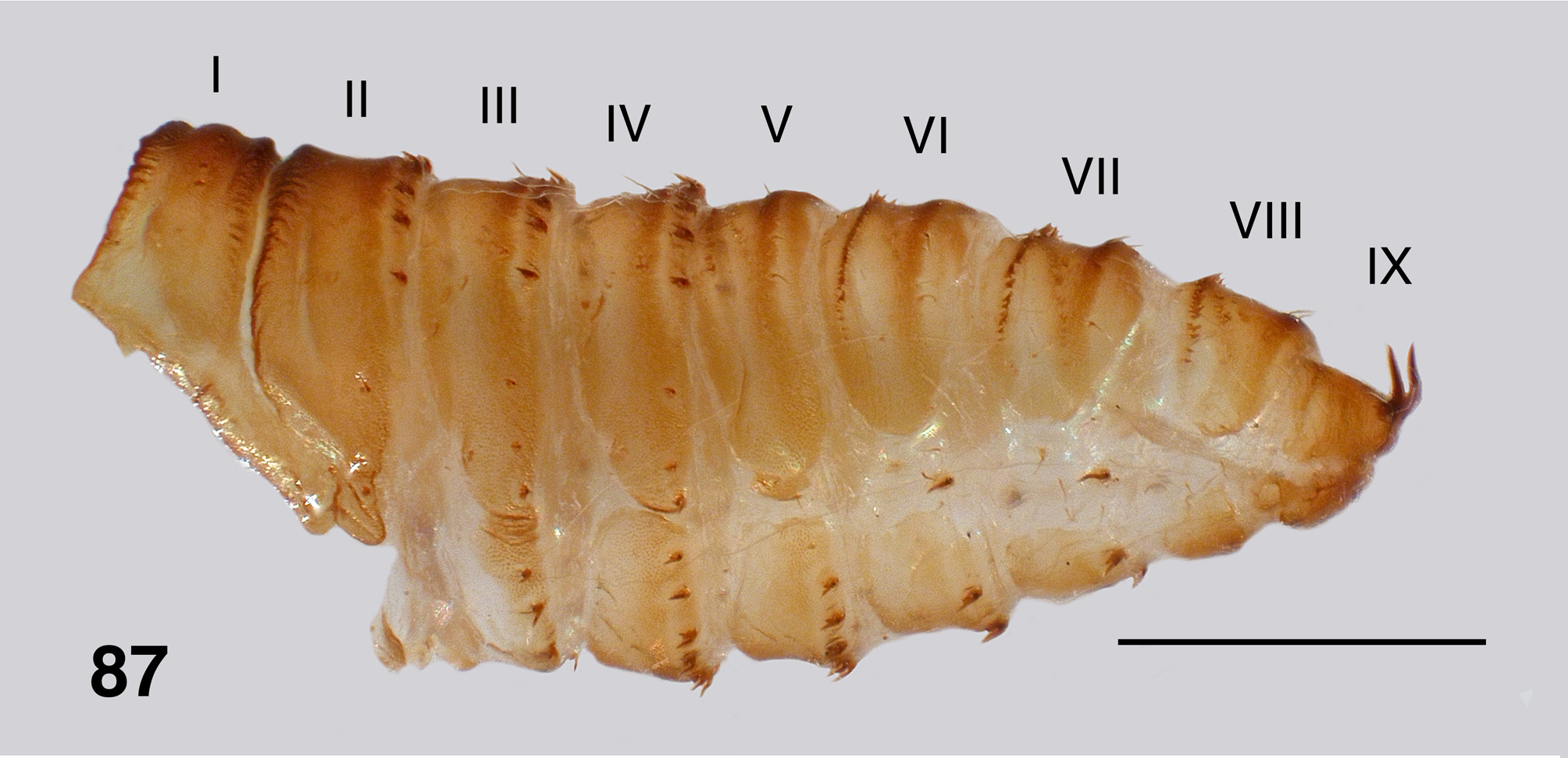
Pupa (numerous specimens, Bartle Frere & Souita Falls). Typically found clumped in large masses (Fig. 80). Body: female length 4.8–5.0 mm ( Fig. 81 View FIGURES 81–86 ), male length 4.5–4.7 mm; brown and yellow, markedly sclerotized with strong cuticle, with or without tubercles. Head: frons of female ( Fig. 82 View FIGURES 81–86 ) broad and apically truncated, with ratios of basal width to vertex width and height 1.0:1.2 and 1.0:1.7 respectively; frons of male ( Fig. 83 View FIGURES 81–86 ) narrow and apically tapered, ratios 1.0:1.8 and 1.0:2.8 respectively; cuticle lightly sculpted but lacking tubercles; dorsolateral frontal setae and facial setae present in males; three sensilla typically present in females, all closely grouped on ocular sclerite beside/beneath antennal sheath, rarely with one on dorsal frons; all setae substantial, without curled tips; antennal sheath of female extended beyond margin of ocular shield, that of male not so extended. Thorax: markedly domed, slightly less so than in A. aurantiaca , smooth, with dorsal trichoid setae short, curved but not curled at tip. Gill ( Fig. 84 View FIGURES 81–86 ): total length 1.2–1.5 mm, with 48–55 light brown filaments arising from 5 or 6 short pale trunks; filaments not markedly tapered, bifurcated at irregular distances from base, ventral filaments directed anteriorly, dorsal filaments directed dorsally, with one or two longer filaments reflexed posteriorly over the thorax; filament surface pseudoannulated throughout ( Fig. 85 View FIGURES 81–86 ). Abdomen ( Fig. 87 View FIGURE 87 ): well sclerotized, especially the anterior tergites; tergites III & IV extended more ventrad than other tergites, separated from sternite by narrow band of striate membrane; segment V with small pleurite present, fused to tergite; other pleurites absent, replaced with minute plates underlying lateral hooks on segments VI & VII; abdomen with minute rounded tubercles, present or occasionally absent—when present sparser on terminal segments. Abdominal armature well developed; tergite I with fine hairs; tergites II–IV each with 4+4 central and 3+3 lateral anteriorly directed long thin recurved hooks (similar to the ventral hooks), lateral smaller than central; tergite V with four hairs and poorly expressed spine comb; tergites VI & VII with well developed spine comb anteriorly and posterior double pair of fine hairs on each side; tergite VIII with small spine comb and a pair of fine hairs posteriorly on each side; segment IX with sharply tapered slightly curved terminal spines, numerous other simple, long curved terminal setae; sternite III with 3+3 anteriorly directed simple recurved hooks, sternite IV with 5+5, sternite V with 5+5, sternite VI with 2+2, and sternite VII with 1+1; pleurites VI & VII with 1+1 anteriorly directed simple recurved hooks.
Cocoon: Length 3.0–4.0 mm, close fitting, often covering whole pupa, or just to half of the thorax, gills extended; irregular weave, less unorganized than others in the genus, silk fibers ( Fig. 86 View FIGURES 81–86 ) light brown and thick, some markedly so and strong; extraneous material from substrate incorporated.
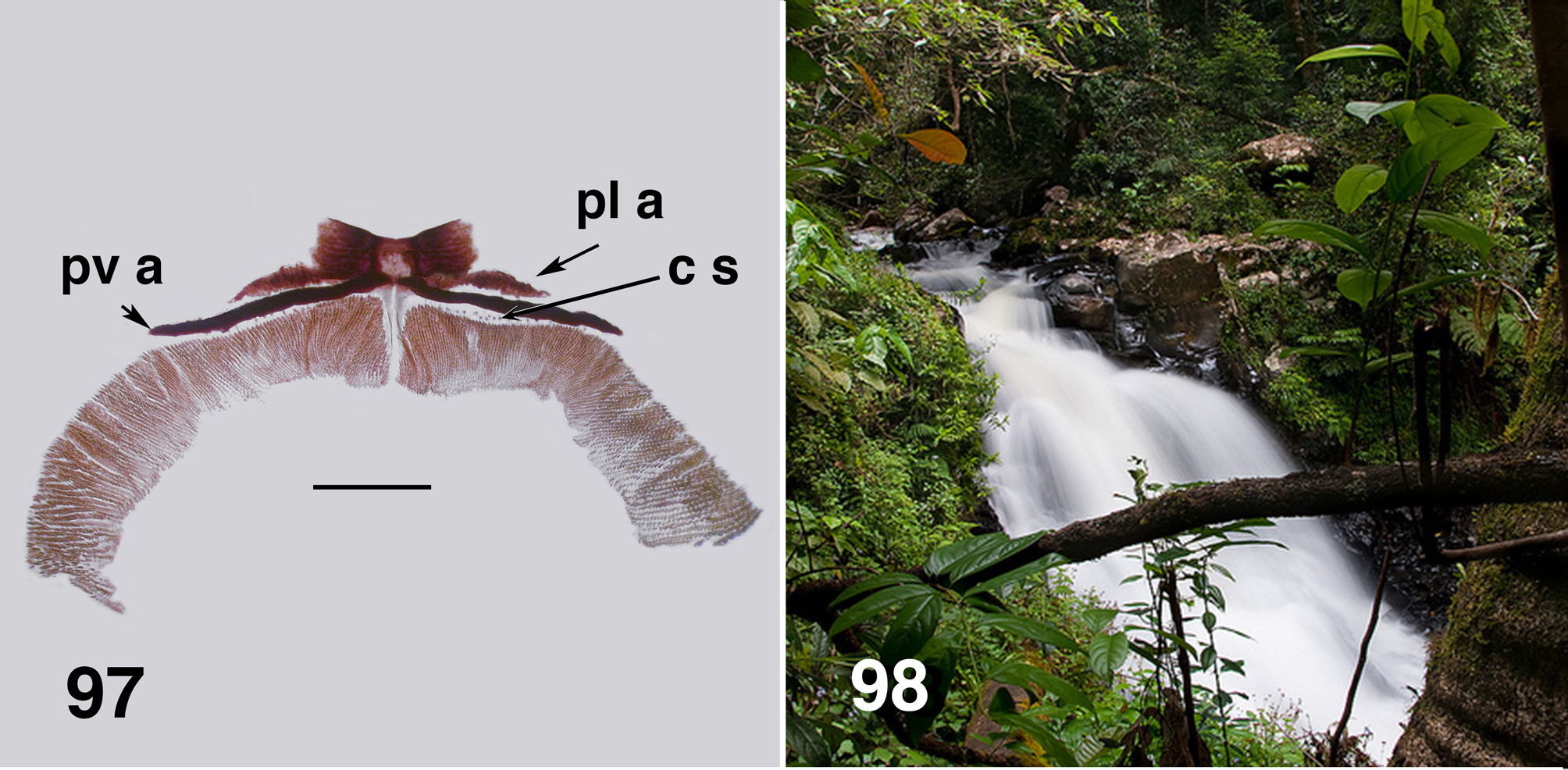
Larva (based on numerous last instar larvae). Body ( Fig. 88 View FIGURES 88–90 ): total length 8.0–11.0 mm, evenly mottled brown, smoothly expanded from the head posteriorly, expanded posteroventrally. Head ( Fig. 89 View FIGURES 88–90 ): relative to body, small, evenly dark brown; head spot pattern poorly developed, but positive; length 0.80–1.15 mm, width 0.73–0.93 mm; distance between antennal bases 0.43–0.55 mm; anterior margins of head tapered anteriorly, straight, broadest posterior to stemmata; ecdysial lines markedly visible, divergent albeit straight until posterior of stemmata, then broadly curved medially; cervical sclerites distinct and finely joined to postocciput, varied; genae markedly dark brown. Antenna: short, not extended to end of labral fan stem; total length 0.30 mm; basal article short and clear proximally, dark brown distally, medial article and distal articles dark brown with marked clear junction, subequal in length; two sensilla at junction markedly pointed; basal article shorter than medial article, proportional lengths of basal, medial, and apical articles 1.0:1.4:1.5; medial article expanded towards distal junction, distal article longer than in A. aurantiacum . Labral fan: stem short and dark brown, ca. 43–50 fine rays, six to ten posterior rays finer than remainder, length ca. 0.66 mm, mid-ray width 0.015 mm; pattern of microtrichia not markedly developed, larger microtrichia longer than ray width, four or five smaller microtrichia interspersed, varied. Mandible ( Fig. 90 View FIGURES 88–90 ): darkly pigmented; short; brushes markedly developed; dorsal margin slightly more curved than in A. aurantiaca ; outer, apical and subapical teeth not accentuated, subequal in length; six to seven spinous teeth, distal two teeth markedly developed (but varied); serration small, often no evidence of sensillum; blade region smoothly convex. Maxilla: palp cone-shaped 1.8–2.0× as long as basal width, darkly pigmented; dense tuft of hair and long spinous hairs at base of palp. Postgenal cleft ( Fig. 91 View FIGURES 91–94 ): moderately developed, shallow, U-shaped, sclerotized with slightly irregular edges and occasionally a small medial projection; posterior tentorial pits small and rectangular in shape; postgenal bridge evenly lighter brown; elongated posteroventral muscles spots not markedly obvious; ratio of hypostoma: genal bridge: postgenal cleft variable—1.0:1.5(1.7):0.5(0.8). Hypostoma ( Figs. 92, 93 View FIGURES 91–94 ): small, darkly pigmented and not markedly extended from head margin, lateral margins gently sloped; teeth in various arrangements; tooth 0 prominent, flanked by teeth 1–3, subequal, 2 & 3 sometime smaller and varied; tooth 4 slightly larger than previous teeth, slightly flanged basally, teeth 5–7 markedly small and difficult to observe, tooth 8 often directed laterally; lateral serrations on hypostoma absent; six to eight substantial, closely-packed hypostomal setae on each side; ventral edge of hypostoma not markedly developed, still, slightly obscuring teeth 5–7. Thorax ( Fig. 94 View FIGURES 91–94 ): anteriorly mottled brown, remainder pale; pupal gill histoblast with 5–7 basal trunks visible, broadly L-shaped, directed ventrally, then sharply posteriorly, broadly rounded with filaments directed anteriorly then dorsally, some bifurcations visible. Prothoracic proleg: strongly developed with distinct L-shaped lateral plates; lateral lappet extensions markedly obvious ( Fig. 95 View FIGURES 95, 96 ), in some localities shorter and broader, or apparently not extended. Abdomen: evenly mottled brown; expanded evenly posteriorly. Ventral tubercles: absent. Anal sclerite ( Fig. 96 View FIGURES 95, 96 , 97 View FIGURE 97 ): complex and variable, anterolateral arms basically just anterior flanges of the medial region, itself various; medial region with the well developed posteromedial space sometimes closed off to form a hole, posterolateral arms from medial region parallel the elongated, substantial posteroventral arms—giving appearance of doubled ventral arms (also in earlier instars); elongated extensions from the medial ends of the posteroventral arms between the dorsal junction of circlet of hooks variable—not well developed. Array (14 or 15) of campaniform (sometimes as markedly short setae) sensilla between the posteroventral arms and circlet of hooks, varied. Rectal papillae: three simple lobes. Posterior circlet: markedly developed and directed slightly ventrally on posterior abdomen; large numbers of hooks, ca. 340 rows of hooks with ca. 50 hooks per row (total ca. 17,000).
Etymology. Named by Mackerras & Mackerras (1950: 172), literally, for the preferred strenuous habitat of larvae—that of high velocity water.
Types. The type locality is Queensland, Cairns, Cascades, Freshwater Creek (S16.9300° E145.6900°). Adult. Coll. Mackerras. ANIC. The female holotype and two male paratypes were examined by LHG-A in 2007. Exact labeling not recorded.
Additional material examined: Two pinned specimens from ANIC were examined by DAC in detail. One, a female from [Little Crystal Cr./ Mt Spec, N.Q./ 6.12.54] [bred out] [ C. strenua / det. J. Prince] [Aust. Nat. Ins. Coll.], the other a gravid female from [Lamington/ Nat. Pk., Qld/ 6-1-. ii.1961 / I. C. Yeo] [Gressitt/ Trap] [Open forest] [ Cnephia nr/ strenua/ det. J. Prince] [Aust. Nat. Ins. Coll.]. Both specimens are now slide mounts in ANIC.
Alcohol material: Large numbers of larvae and pupae from collections by P. & H. Zwick, made mainly in the 1970’s. [ ANIC Database No. 29 026508–523, 29 026525–528, 29 026530–531]. Also, considerable material of all stages collected, in the main, by JKM, 1996. [ UASM #/370803–370806, 370827, 370828]. Slide mounts: All stages [ UASM #/370749–370781].
Bionomics. Both Mackerras & Mackerras (1950:170) and Colbo (1974) noted that little was known about this species. It appears, however, to be multivoltine since later instar larvae plus pupae have been found from October to June—an Austral early summer to autumn species. Colbo (1974), though, noted that for SE Queensland the season was April to November—an extended ‘winter’ species? For Northern Queensland the season was from September to December, a ‘spring’ species. There is no useful information on water temperatures.
There is no record of this species biting humans and no adults have been taken in the field. For the Souita Falls population, however, females possess teeth on both sides of the mandible (unique in the genus), have poorly developed abdominal tergites, maxillary palp sensory vesicle well expressed and a substantial tarsal claw basal tooth, characters that point to probable ornithophily ( Shewell, 1955; Sasaki et al., 1985; Adler et al., 2004).
The number of eggs from the gravid Lamington specimen, ca. 600, is towards the upper numbers of eggs known for simuliids ( Crosskey, 1990: 462) and again, suggests blood feeding rather than autogeny.
Larvae and pupae are often clumped, with the latter forming mats of overlapping specimens, most of similar developmental stage. That aspect of the biology is discussed later. Mackerras & Mackerras (1950: 173) noted that larvae are found in extremely fast flow to the point where they are difficult to collect. Pupae were in lower velocity. Recent collections are in full agreement.
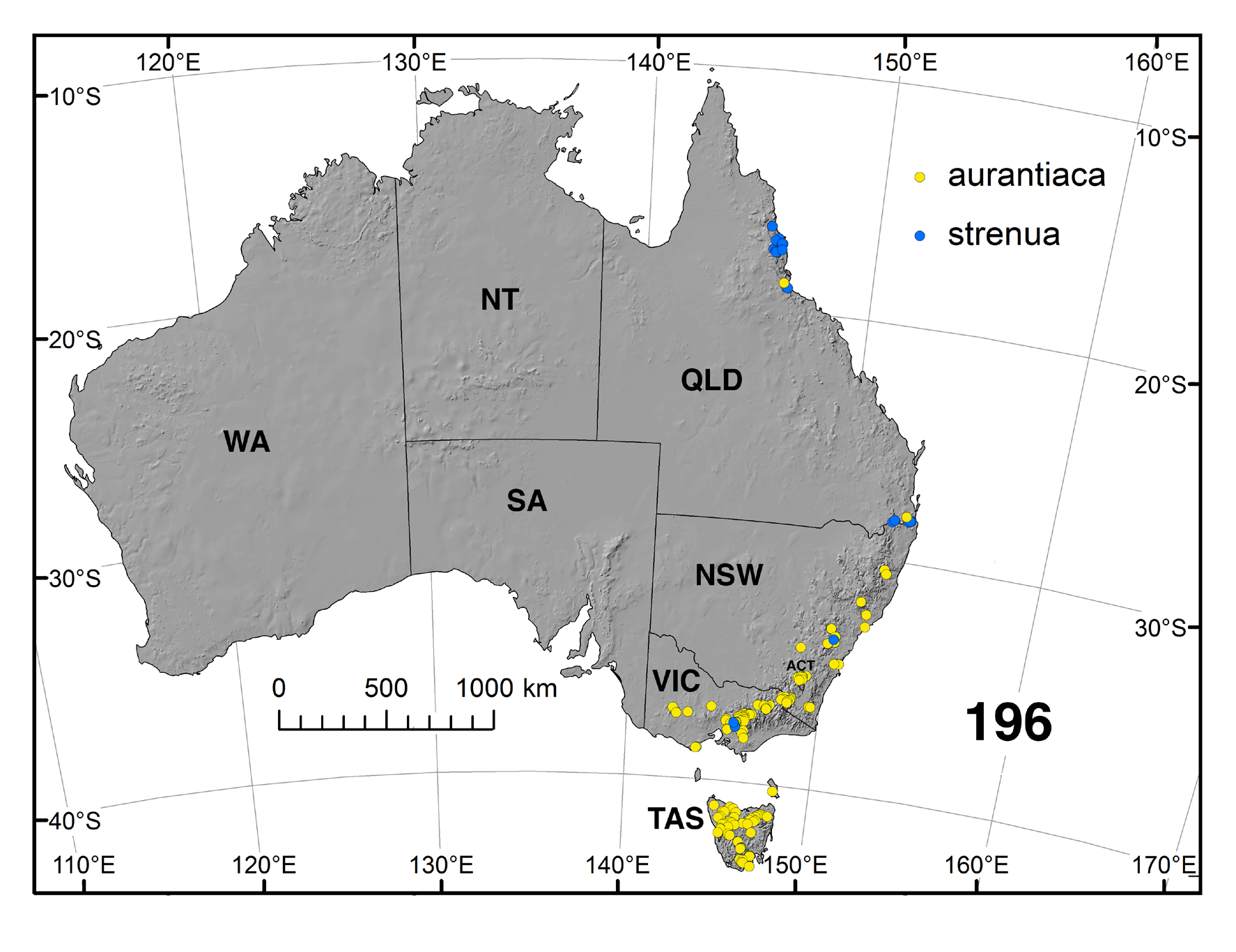
Distribution ( Fig. 196 View FIGURE 196 ). Austrocnephia strenua is the most northerly-distributed member of the genus.
Queensland: Mossman, Mossman Gorge, S16.4748° E145.3432°. 18 Oct. 2002, Larvae, pupae. Coll. Zwick (ANIC). Mossman Gorge, near Cairns, S16.4748° E145.3432°. 20 Feb. 1973. Larvae, pupae. Coll. Zwick (ANIC). Mossman Gorge, Rex Creek, main stream, S16.4695° E145.3291°. 26 Mar. 1992. Larvae. Coll. H. & P. Zwick (ANIC). Mossman Gorge, S16.4748° E145.3432°. Coll. Cantrell ( Colbo 1974). Redlynch, nr. Cairns, Crystal Cascade, tributary, S16.9617° E145.6794°. 5 Apr. 1997, Larvae, pupae. Coll. Zwick (ANIC). Freshwater Creek, nr. Cairns, S16.9617° E145.6794°. 5 Jan. 1973, Larvae. Coll. B. Cheesman (ANIC). Cairns, Freshwater Creek, S16.9617° E145.6794°. 15 Sept. 1949. Larvae. Coll. Mackerras (UASM, ANIC). Malandra, The Crater, S17.2800° E145.6200°. 27 Jun. 1971. Larvae. Coll. E. Riek (ANIC). Ringrose National Park, Atherton, S17.4200° E145.4800°. Coll. Cantrelli ( Colbo 1974). Malandra, upper Barron R., Dinner Falls, S17.4331 E145.4833°. 24 Oct. 2002. Larvae. Coll. Zwick (ANIC). Mt. Bartle Frere, Josephine Falls, S17.4420° E145.8600°. 6 April 1997. 10 Dec. 1997. Larvae, pupae. Coll. Zwick (ANIC). Souita Falls, nr Millaa Millaa, S17.5600° E145.6500°. 6 Mar. 2001. Larvae, pupae. Coll. H. & P. Zwick (ANIC). Atherton Tablelands, Souita Falls, Middlebrook Rd., ex. Old Palmerston Rd, S17.5600° E145.6500°. 18 Oct. 1996. Larvae, pupae, adults. Coll. J. K. Moulton (UASM). Atherton Tablelands, nr. Millaa Millaa, S17.5000° E145.600°. 19 Oct. 2002. Larvae, pupae. Coll. Zwick (ANIC). Palmerston Hwy., nr. McHugh Bridge, Little Beatrice River, S17.5521° E145.6092°. 22 Feb. 1973. Larvae. Coll. Unknown (ANIC). Atherton Tablelands, Emerald Ck., S17.0580° E145.5470°. 1 Jan. 1998. Larvae. Coll. A. Zwick (ANIC). Fishery Falls, S17.1800° E145.8800°. 29 Jun. 1971. Coll. E. Riek (ANIC). South of Gordonvale, Behana Gorge, Clamshell Falls, S17.1894° E145.8233°. 28 Mar. 1997. Larvae. Coll. Zwick (ANIC). Behana Gorge, nr Cairns, S17.1650° E145.8320°. 12 Feb. 1973. Larvae. Coll. Zwick. 29 Jun. 1971. Pupae. Coll. E. Riek (ANIC). Mt. Bartle Frere, Josephine Falls, S17.4328° E145.8597°. 10 Oct. 1997. Larvae, pupae. Coll. A. Zwick; 24 Mar. 1992. Coll. H. & P. Zwick (ANIC). Little Crystal Creek, 45 m N of Townsville, S18.979° E146.197°. (November-December) Mackerras & Mackerras (1950). Paluma Range, Mt. Spec Road, Little Crystal Creek, S19.0154° E146.2656° ( Bugledich 1999); 19 Mar. 1997, Larvae. Coll. Zwick. 12 Apr. 2002, Coll. A. Zwick (ANIC). Springbrook National Park, Purling Brook Falls, S28.1898° E153.2709° ( Colbo 1974). Lamington National Park, S28.2200° E153.1500°. Bugledich (1999: 328). Lamington Plateau, Elabana Falls, S28.2500° E153.1497°. 26 December 1954. Coll. Mackerras (ANIC). Wilsons Peak, Teviot Brook, S28.2500° E152.4800°. 23 Apr. 1971. Larva, pupa. Coll. M.H. Colbo (ROM) Springbrook, Wilsons Peak, Condamine River, near Queen Mary Falls, S28.3400° E152.3735°. April–November. ( Colbo 1974). Lamington Nat. Park, Binna Burra, Lower Ballanjui Falls, S28.2166° E153.2333°. 9 Mar. 1997. Larvae, pupae. Coll. Zwick (ANIC). Wilsons Peak, Teviot Brook, S28.2500° E152.4800°. 23 April 1971. Larvae, pupa. Coll. Zwick (ANIC).
New South Wales: Blue Mountains National Park, Bridal Veil Falls, Govetts Leap , S33.6333° E150.3129°. 5 Nov. 18 1998; Oct. 2014. Larvae, pupae. Coll. D.A. & R.E.G. Craig ( UASM) GoogleMaps .
Victoria: Warburton, Cement Creek , S37.7239° E145.7542°. 1 April 1972. Larvae. Coll. Zwick ( ANIC) GoogleMaps . Narbethong, Anderson Lane, Stony Creek , S37.54607° E145.6546°. 22 Oct. 2014. Reared males. Coll. D.A. & R.E.G. Craig ( UASM) GoogleMaps .
Remarks. Austrocnephia strenua is a distinctively large species and was so noted by Mackerras & Mackerras (1950: 170). Apart from the large size, in particular that of the larvae, the adults are brightly coloured on the thorax and have pigmentation on the wings. The male eyes have reduced rows of large upper ommatidia, thought at the time to be unique in Australian Simuliidae ( Mackerras & Mackerras, 1950: 170) . That, however, was mainly because most simuliid species in Australia were then described from female adults. This feature of the males is now known to be common for Austrocnephia . Large upper ommatidia are reminiscent of some species of Gomphostilbia Enderlein , such as Simulium (G.) laciniatum Edwards, of Fiji. The markedly domed thorax in both male and female A. strenua adults is also well expressed elsewhere in the genus.
There is considerable variation in the degree of tuberculation on pupal abdominal tergites. We illustrate the condition from Bartle Frere ( Fig. 87 View FIGURE 87 ) where it is well expressed. Tubercles, however, are absent from Lamington National Park material and elsewhere. Similarly there is variation in the development of the spinous teeth of the larval mandible. Colbo (1974: 68) noted that South Queensland larvae differed from northern larvae in having a different pattern of microtrichia on the labral fan rays, plus the prothoracic proleg lappet expression was slightly different. The variation in character states in different populations ( Fig. 196 View FIGURE 196 ) of A. strenua , such as teeth on the one or other sides of the female mandible, differences in the apex of the male ventral plate ( Fig. 76 View FIGURES 75–79 vs. 78) from that illustrated by ( Mackerras & Mackerras, 1950. Their Fig. 3 View FIGURES 1–6 ), pupal gill filament number, tuberculation on the pupal abdomen, serrations on the larval mandible, and those mentioned previously, well indicate that as presently defined, A. strenua is likely a complex of closely related entities. There are, indeed, considerable distances between different populations, particularly those to the south. Since, however, we lack complete material from such populations, we refrain from designating new taxa.
Palmer & Craig (2000) examined total number of hooks comprising the posterior circlet in larvae of simuliids in relation to velocity of preferred habitat. Such a correlation was noted by Mackerras & Mackerras (1950: 172) for A. strenua and for other simuliid larvae by Crosskey (1990: 110), Adler et al. (2004: 58) and Figueiróa et al. (2015), amongst others. Inhabiting extreme velocity flow, an expectation would be that A. strenua might perhaps approach the record number of hooks known for simuliid larvae. While numerous (ca. 17,200), hooks do not approach numbers exhibited by larvae of Freemanellum Crosskey species ( Palmer & Craig, 2000: 202), or of those of Daviesellum Takaoka & Adler ( Takaoka & Adler, 1997: 18), larvae of which inhabit jets of water impinging on rocky surfaces.
There are a number of other character states of larvae and pupae that appear to directly relate to the extreme high velocity habitats, e.g., Souita Falls (Fig. 98)—firstly, the substantial nature and size of the larvae. Mackerras & Mackerras (1950: 172) commented that the body was very muscular. The anal sclerite is markedly developed with the posteroventral arms paralleled by the posterolateral arms arising from the medial region of the anal sclerite. Further, there are numerous campaniform sensilla between the posterior arm and the circlet of hooks—in other simuliid larvae they generally number four or so. The circlet of hooks is also markedly developed with number of hooks considerable and expression substantive; again fitting well with the extreme habitat. Further, unique to Austrocnephia , and in particular A. strenua , is possession of two anterolateral structures on the prothoracic proleg ( Fig. 95 View FIGURES 95, 96 ). As noted previously, these were termed ‘palp-like processes’ by Mackerras & Mackerras (1950: 172), and ‘lapets’ by Colbo (1974: 69) —we use the term ‘lappets’. The proleg also has well expressed L-shaped lateral sclerites and the lappets arise from the internal apices of those, but while the lappets are often pigmented, they are not well sclerotised. Neither does there appear to be any muscular attachment. What function these lappets serve is unknown. Less well developed lappets can occasionally be observed in A. aurantiaca , A. orientalis and A. tonnoiri , but have not been observed in larvae of A. fuscoflava . Homologies of the lappets is moot. Van Oye (1936) illustrates a possible homolog, albeit on the prothoracic proleg of Simulium larvae. For pupae of the aurantiaca species-group, cuticle is well sclerotized and cocoon silk markedly strong.
Forming aggregations for aquatic organisms in high velocity flow is a well-known ploy to ameliorate drag forces (e.g., Nowell & Jumars, 1984: 317; Craig, 2003: 1087). In such an arrangement the boundary layer of the water lifts and flows over the aggregation rather than around or between individuals, thereby reducing drag and is known as ‘skimming flow’. However, consequences of such behaviour are probably that for larvae, packed close together, individuals must be elongated and labral fans well expressed to access particulate matter. Indeed, an expanded posteroventral abdomen ( Fig. 88 View FIGURES 88–90 ) appears characteristic of simuliid larvae that inhabit fast flows (e.g., Craig, 1987a: 396). Similarly, clumped pupae achieving skimming flow would still require the gills to be exposed to flow and thence the cocoons are stacked, with only the posterior of the abdomen attached to the substrate, as noted here for A. strenua and some other members of the genus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Austrocnephia strenua ( Mackerras & Mackerras 1950 )
| Craig, Douglas A., Currie, Douglas C., Gil-Azevedo, Leonardo H. & Moulton, John K. 2019 |
Paracnephia strenua.
| Adler, P. H. & Crosskey, R. W. 2008: 26 |
Paracnephia strenua.
| Crosskey, R. W. & Howard, T. M. 2004: 10 |
Cnephia ’ strenua
| Moulton, J. K. 2003: 47 |
| Moulton, J. K. 2000: 110 |
Paracnephia strenua
| Crosskey, R. W. & Howard, T. M. 1997: 18 |
strenua
| Crosskey, R. W. 1989: 222 |
Cnephia
| Crosskey, R. W. 1987: 443 |
Cnephia strenua
| Rothfels, K. H. 1979: 522 |
Cnephia strenua
| Mackerras, M. J. & Mackerras, I. M. 1955: 105 |
| Mackerras, M. J. & Mackerras, I. M. 1950: 170 |