Paranaspides williamsi, Ahyong & Schwentner & Richter, 2017
|
publication ID |
https://doi.org/ 10.3853/j.2201-4349.69.2017.1679 |
|
publication LSID |
lsid:zoobank.org:pub:BD74A0FE-CB17-4D7C-8595-3912F0406AA7 |
|
persistent identifier |
https://treatment.plazi.org/id/613B9B3D-7EE8-4A00-8DD2-A4411956B170 |
|
taxon LSID |
lsid:zoobank.org:act:613B9B3D-7EE8-4A00-8DD2-A4411956B170 |
|
treatment provided by |
Felipe |
|
scientific name |
Paranaspides williamsi |
| status |
sp. nov. |
Paranaspides williamsi sp. nov.
urn:lsid:zoobank.org:act:613B9B3D-7EE8-4A00-8DD2-A4411956B170
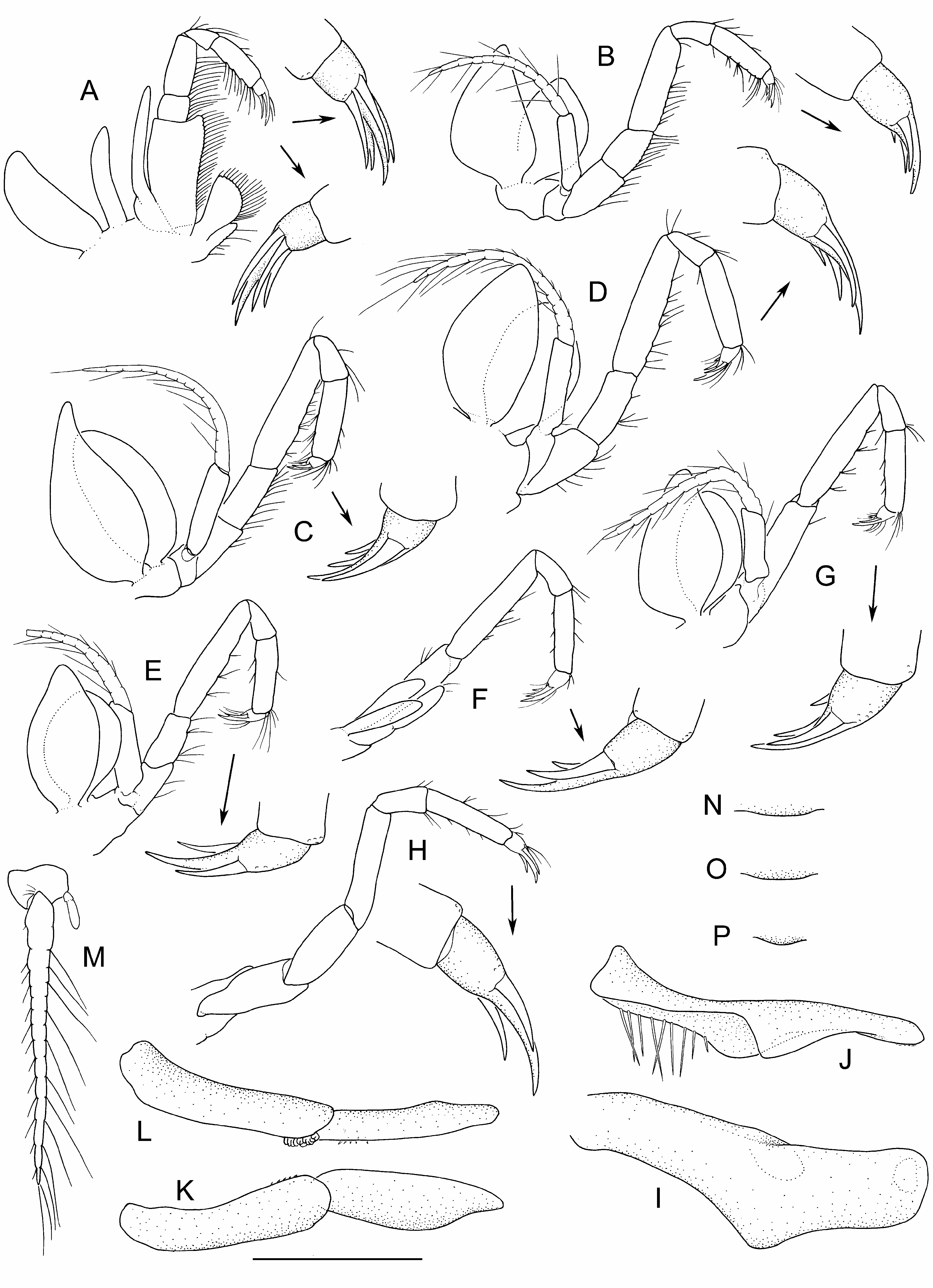
Figs 4–8 View Figure 4 View Figure 5 View Figure 6 View Figure 7 View Figure 8 , 9B View Figure 9 , 10 View Figure 10
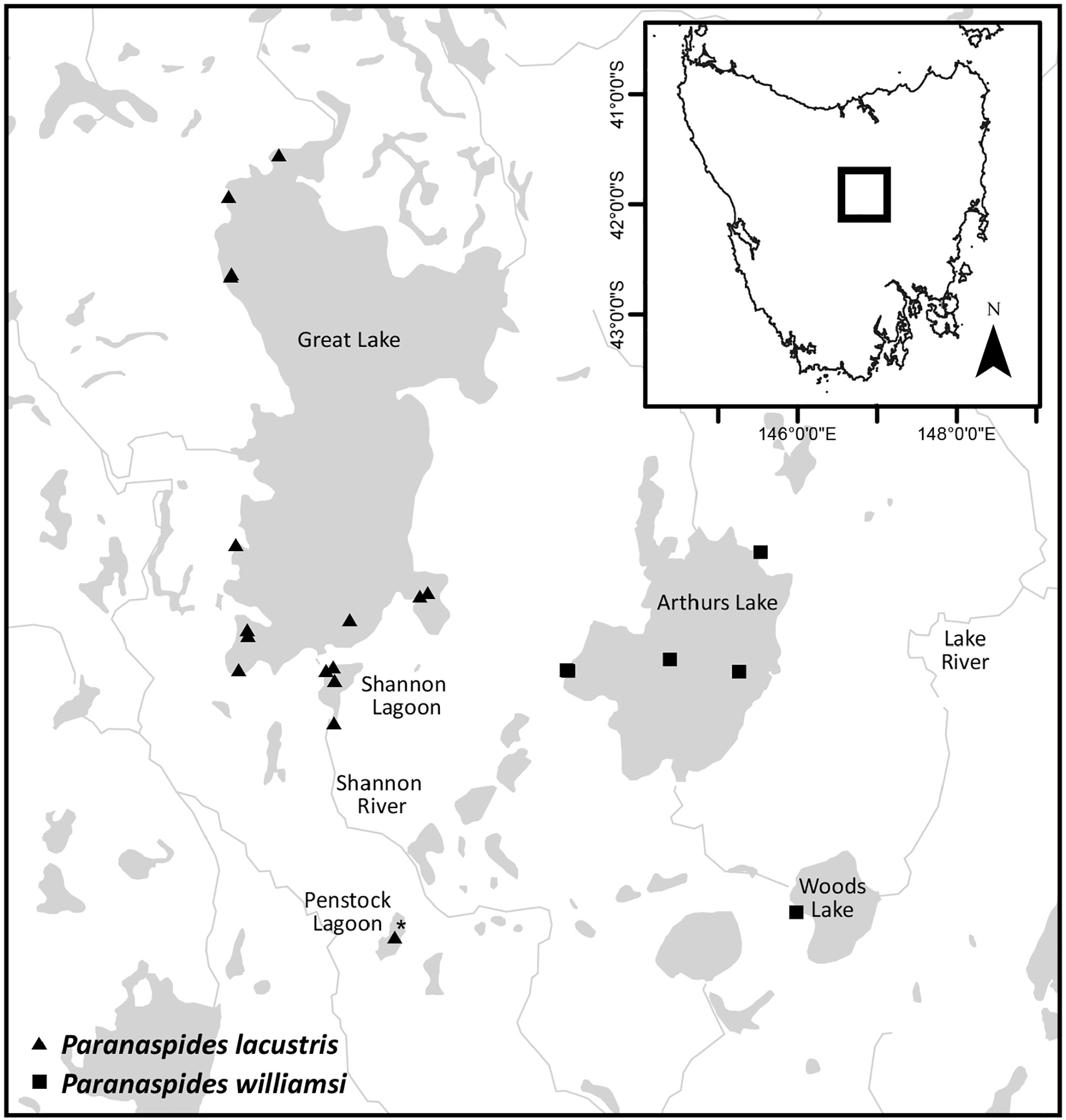
Paranaspides lacustris . — Fulton, 1982: 23, 25, fig. 1 [Woods Lake, Arthurs Lake, Lake River only]; 1983: 776, tab. 1 [Arthurs Lake].
Holotype: TMAG G8244 View Materials , male (12 mm), Arthurs Lake , Pumphouse Bay, near pumping station, 41°59'16.3"S 146°51'44.8"E, netted from weeds, <1 m, coll. S. Richter & C. Wirkner, 28 February 2006 GoogleMaps . Paratypes: AM P99963, 3♂♂ (11–12 mm), 6♀♀ (13–14 mm), collected with holotype GoogleMaps ; AM P100414 , 3♀♀ (11–12 mm), Pumphouse Bay , near pumping station, 41°59'15"S 146°51'42"E, Nitella beds, 0.7 m, light trap, coll. S. Ahyong, C. Hoepel, M. Reinhardt, S. Richter, 10 March 2017 GoogleMaps ; QVM 10 View Materials :49058, 1♀ (15 mm), southern East Lake , Arthurs Lake [41°59.30'S 146°57.19'E], coll. W. Fulton, 17 May 1977 GoogleMaps .
Other material examined. Arthurs Lake: AM P100413 , 4♂♂ (11 mm), 1♀ (11 mm), Pumphouse Bay , near pumping station, 41°59'15"S 146°51'42"E, Nitella beds, 0.2–0.7 m, hand net, coll. S.Ahyong, C.Hoepel, M.Reinhardt, S. Richter, 8 March 2017 GoogleMaps ; ZSRO CR23 , 3♂♂ (12–13 mm), 2♀♀ (11–12 mm), Pumphouse Bay , near pumping station, 41°59'15"S 146°51'42"E, Nitella beds, 0.2–0.7 m, netted, coll. S. Ahyong, C. Hoepel, M. Reinhardt, S. Richter, 8 March 2017 GoogleMaps ; QVM:10:49059, 1 damaged ♂, 1♀ (15 mm), 1 exoskeleton, Sand Lake , Arthurs Lake [41°56.46'S 146°57.88'E], coll. W. Fulton, 29 June 1977 GoogleMaps ; QVM 10 View Materials : 49057, 1♂ (16 mm), 2 damaged ♀♀, southern East Lake , Arthurs Lake [41°59.30'S 146°57.19'E], coll. W. Fulton, 19 April 1977 GoogleMaps .
Woods Lake: QVM 10 View Materials :49161, 10♂♂ (14–16 mm), 10♀♀ (13–16 mm), west shore Woods Lake , [42°05.50'S 146°59.82'E], coll. W. Fulton, 22 July 1977 GoogleMaps .
Description. Pleonite 6 lower mid-lateral surface with arcuate row of 3–5 (usually 4) prominent, well-spaced spines; posterolateral angle bispinous (rarely unispinous); posteroventral angle anterior to uropod articulation with cluster of 6–14 spines.
Antennule mesial (accessory) flagellum 0.1–0.2 × body length (17 articles in holotype); lateral flagellum 0.4 × body length (42 articles in holotype).
Antennal flagellum 0.3–0.4 × body length (40 articles in holotype); protopod coxa with splayed row of 6–9 spines on lateral margin, basis with 2 lateral spines.
Labrum anterior proximal surface swollen medially, without median point.
Thoracopod 1 (maxilliped) merus slightly tapering distally, length twice length of ischium.
Pleopods 3–4 endopod always present in adults; pleopod 5 endopod rarely present. Adult male pleopod 1 margin of dorsodistal half straight to faintly concave. Male pleopod 2 endopod distal article with distoventral surface broadly concave.
Uropodal protopod with cluster of 1–4 posterolateral spines. Uropodal exopod elongate, spatulate; lateral margin between incurved anterolateral margin and distolateral spine row, straight or faintly or faintly concave, with 0–7 minute, widely spaced setae; distolateral spine row of 9–14 fixed graded spines; spine row length 0.4–0.7 × length of straight portion of preceding exopod margin.
Colour in life ( Fig. 9B View Figure 9 ). Body transparent, covered in dull red and brown chromatophores forming diffuse transverse bands across pereon and pleon, most pronounced and darkest across pereonites 2–3, 7 and anterior half of pleonite 6; with dark-brown; cephalothorax with red brown patch on lateral surface behind cervical groove. Antennular peduncle article 1 transparent with scattered spots and dark midline; article 2 transparent with longitudinal brown midline and brown mesial margin; article 3 transparent, with partial pigmentation. Scaphocerite transparent. Eyestalks red brown. Pereopods and pleopods translucent pale brown. Tailfan transparent with scattered brown spots, densest distally.
Measurements. Male (n = 23) 11–16 mm; female (n = 26) 11–16 mm.
Remarks. Paranaspides williamsi sp. nov. differs from P. lacustris in the following features: the proportionally shorter merus of the maxilliped (length twice the width in the new species versus 2.5–3 times length in P. lacustris ), the proportionally longer spine row on the uropodal exopod (about half or longer versus one-third length of the preceding straight, unarmed lateral margin), and subtle differences in the adult male pleopods 1 and 2. The adult male pleopod 1 distodorsal margin is straight or, at most, faintly concave in P. williamsi , rather than noticeably to strongly concave in P. lacustris . The distoventral margin of the distal article of adult male pleopod 2 is concave in P. williamsi , straight in P. lacustris . In both species of Paranaspides , the number of pleopodal endopods is variable and overlapping, but with different ranges. In P. lacustris , the pleopodal endopods are usually present on pleopods 1–4, but may be absent on pleopod 4, even in adults. In P. williamsi , endopods are present on pleopods 1–4, and in one specimen, also on pleopod 5 (damaged adult male, QVM 10:49050). Both P. lacustris and P. williamsi appear to mature at a similar size (c. 10–11 mm), although they differ in maximum known body length (25 mm versus 16 mm, respectively). Whether this size difference reflects reality or limited sampling remains to be determined. Colour-in-life ( Fig. 9 View Figure 9 ) is similar between P. williamsi and P. lacustris , though in the latter, the distal article of the antennular peduncle is solid dark brown versus being partially pigmented, and transverse banding is uniform rather than darkest and most pronounced on pereonites 2–3 and 7, and on pleonite 6.
The distributions of the two species of Paranaspides precisely parallel those of a cognate pair of freshwater fishes, Paragalaxias , distributed in Great Lake-Shannon Lagoon- Penstock Lagoon ( Paragalaxias eleotroides McDowall & Fulton, 1978 ) and Arthurs Lake-Woods Lake-Lake River below Woods Lake dam ( Paragalaxias mesotes McDowall & Fulton, 1978 ) (McDowall & Fulton, 1978; Fulton, 1982). Great Lake and Arthurs Lake are geographically close (c. 6 km), and, given the shared cognate species pairs, both lake systems probably shared a common system in the past. Great Lake and Arthurs Lake now occupy different drainages, with the former draining to the southeast towards Hobart via the Shannon River and Derwent River, and the latter draining northeast towards Launceston via Woods Lake, the Lake River and then the Tamar River (McDowall & Fulton, 1978). Great Lake and Arthurs Lake are believed to be preglacial and apparently escaped glaciation during the Pleistocene ( Davies, 1974; Kiernan, 1990; Andrew, 2005). McDowall & Fulton (1978) hypothesised that the divergence between the respective cognates of Paragalaxias might also be pre-Pleistocene. Molecular divergence estimates of Central Plateau Paragalaxias (c. 3–10 ma) ( Waters et al., 2000) corroborate the hypothesised pre-Pleistocene divergence of selected Great Lake and Arthurs Lake taxa and isolation of drainages proposed by McDowall & Fulton (1978). Given the striking parallels with species of Paragalaxias , the divergence of Paranaspides might also be pre-Pleistocene.
Distribution. Arthurs Lake, Woods Lake and the Lake River below Woods Lake dam; 738–952 m asl.
Conservation status
Paranaspides lacustris was abundant in Great Lake amongst the extensive stands of nearshore charophyte algal beds prior to its stepwise modification and damming since the c. 1920s, leading to significant population declines ( Manton, 1930). These algal beds typically occur only down to about 10 m depth given light attenuation, so significant increases in lake level are particularly deleterious, especially given the probable univoltine life-cycle making loss of a yearclass difficult to recover from. Although preyed on by trout ( Richards et al., 2015), the more significant threat to P. lacustris is probably habitat loss caused by changing lake levels. Major reductions in P. lacustris populations observed in the 1920s, 1930s, 1960s and 1970s are associated with progressive artificial increases in lake level ( Wells et al., 1983). Subsequent dam modifications further increased the lake level several times through to the 1980s (Davies & Fulton, 1987; Bonham, 2006). Since the 1920s, P. lacustris 272 Records of the Australian Museum (2017) Vol. 69
has seldom been found in significant numbers in Great Lake, with the frequent raising and lowering of lake levels for hydroelectric operations believed to retard establishment of the littoral vegetation essential as habitat ( Horwitz, 1990).
Little is known of the current population size and dynamics of either species of Paranaspides , so conservation assessments have relied largely on area of occupancy and the limited number of locations at which either species occurs. Paranaspides lacustris is currently assessed by the IUCN Red List of Threatened Species as Vulnerable (D2) ( Inland Water Crustacean Specialist Group, 1996) based on its limited area of occupancy, few known locations, and in being prone to the effects of hydroelectric operations. With Arthurs and Woods Lakes now excluded from the range of P. lacustris , the area of occupancy is reduced, though the Vulnerable D2 assessment would remain applicable. Paranaspides williamsi , being restricted to Arthurs and Woods Lakes (and the Lake River immediately below the Woods Lake dam) has a limited area of occupancy and occurrence at no more than three locations. The dependence of P. williamsi on aquatic vegetation (charophyte and macrophyte beds) indicates an area of occupancy in Arthurs Lake of 0.63–8.3 km 2 depending on water level ( Lobdale, 2011). Although the proportion of vegetated habitat of Woods Lake is not known, the total surface area is approximately 1.2 km 2 so the total area of occupancy of P williamsi (both lakes combined) would not exceed 9.5 km 2. Like P. lacustris , P williamsi is also subject to artificial lake level fluctuations and stochastic events given its very narrow range. As such, the conservation status of P. williamsi under IUCN Red List categories would also correspond to Vulnerable D2. Our efforts to sample P. williamsi in Woods Lake in March 2017, however, were unsuccessful and no other recent collections are presently available. Therefore, establishing the population status of P. williamsi in Woods Lake should be prioritized, especially given the sharp decline in Paragalaxias mesotes observed in Woods Lake over the past two decades (TSSC, 2016). If the Woods Lake population of P. williamsi has also significantly declined, it might require a higher level of protection.
Neither species of Paranaspides is currently listed on either the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 or the Tasmanian Threatened Species Protection Act 1995 ( Bonham, 2006). It is noteworthy, however, that the galaxiid fishes Paragalaxias eleotroides and Paragalaxias mesotes , under both the Commonwealth Environment Protection and Biodiversity Conservation Act 1999 and the Tasmanian Threatened Species Protection Act 1995, are currently assessed as vulnerable and endangered, respectively (TSS, 2006). Given that these species of Paragalaxias parallel the species of Paranaspides in distribution, habitat requirements and in similar threats, they may warrant a similar conservation status under Tasmanian and Commonwealth jurisdictions. Since key proposed conservation priorities for Paragalaxias emphasize mitigating habitat deterioration and loss (TSSC, 2014, 2016), their adoption could also benefit Paranaspides .
ACKNOWLEDGMENTS. Thanks are due to Sammy De Grave and ( OUMNH), Andrew Hosie ( WAM), Kirrily Moore ( TMAG), Judy Rainbird ( QVMAG), and Jo Taylor ( NMV) for the loan of specimens. Karen Reed and Rafael Lemaitre are thanked for their hospitality at the USNM in 2016, and Mark Carnley and Sammy De Grave ( OUMNH) are thanked for facilitating the transfer of type material of P. lacustris to the Australian Museum. Likewise, D. Christopher Rogers and Rachael Peart are thanked for constructive reviews of the manuscript. For our fieldwork in 2017, we gratefully acknowledge the assistance of Alastair Richardson (University of Tasmania), Mike Driessen ( Department of Primary Industries , Parks, Water and Environment, Tasmania), and Stefan Eberhard (Subterranean Ecology Pty Ltd) for facilitating our activities in Tasmania, and Simon Talbot (Institute for Marine and Antarctic Studies, University of Tasmania) for the loan of diving equipment and air compressor; specimens were collected under permit no. TFA 17038 granted by the Tasmanian Department of Primary Industries , Parks, Water and Environment. We also thank Christian Wirkner, Marian Reinhardt and Christoph Hoepel (Universität Rostock) for assistance in the field and Jessica O’Donnell for creating Fig. 10 View Figure 10 . This study was partially funded by a grant from the Australian Biological Resources Study. Collecting in 2017 was funded by the German Science Foundation ( DFG RI 837 /22-1). This is a contribution from the Australian Museum Research Institute .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Paranaspides williamsi
| Ahyong, Shane T., Schwentner, Martin & Richter, Stefan 2017 |
Paranaspides lacustris
| Fulton, W 1982: 23 |