Andinobates victimatus, Márquez, Roberto, Mejía-Vargas, Daniel, Palacios-Rodríguez, Pablo, Ramírez-Castañeda, Valeria & Amézquita, Adolfo, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4290.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:66A83CED-6200-4758-A679-39B5A0656B38 |
|
DOI |
https://doi.org/10.5281/zenodo.6008979 |
|
persistent identifier |
https://treatment.plazi.org/id/038D8789-3575-D921-FF76-7896FEB4F98B |
|
treatment provided by |
Plazi |
|
scientific name |
Andinobates victimatus |
| status |
sp. nov. |
Andinobates victimatus View in CoL sp. nov. Figures 2 View FIGURE 2 and 4 View FIGURE 4 A–K. Table 1.
Holotype. An adult female (ANDES-A 3686) collected by Daniel Mejía-Vargas in Chichiridó, Dabeiba, Antioquia (7.02°, -76.38°), located in the Llorona Canyon, at 280 meters above sea level (m.a.s.l.).
Paratypes. Five adults (4 males and 1 female; ANDES-A 3684, 3685, 3687–3689), collected at the type locality, along with the holotype.
Etymology. From the Latin victimatus (the victimized); noun in apposition. The Urabá region, where this species occurs, has historically been flailed by Colombia’s longstanding armed conflicts, perhaps more than most other regions of the country, leaving a virtually countless trail of innocent victims. We name this species in honor and remembrance of all these victims.
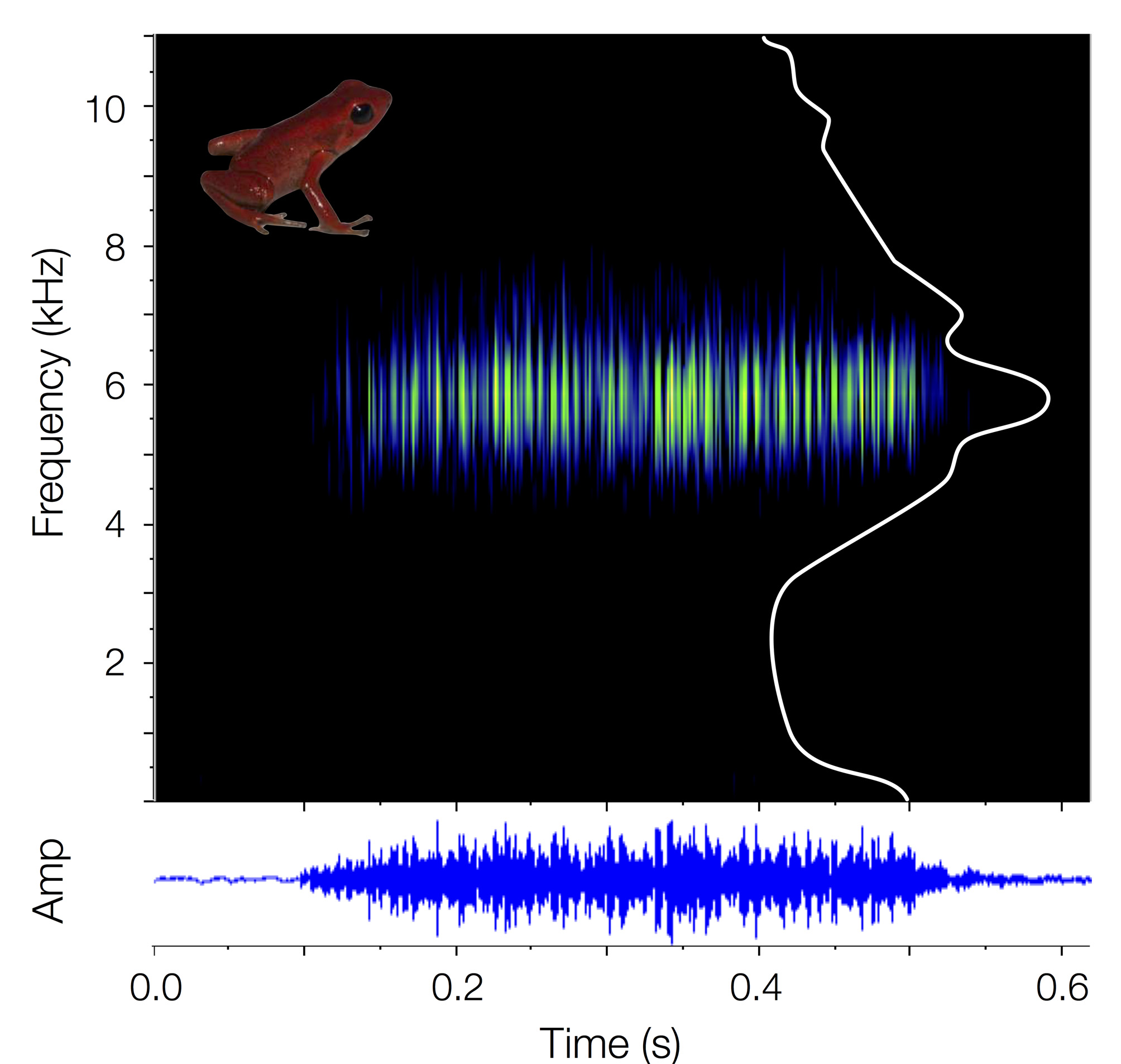
Definition and diagnosis. Andinobates victimatus sp. nov. is a minute (SVL mean = 14.23 ± SD = 0.67 mm; Table 1) dendrobatid frog, characterized by having homogeneous scarlet red dorsal coloration, which extends throughout the limbs. Fingers and toe tips are light metallic gray to metallic gray. The venter and flanks are also scarlet red, with patches degrading into vermilion and finally black, forming irregular blotches ( Fig. 4 View FIGURE 4 A–F). The skin is slightly granular, with granularity increasing towards the urostyle and hind limbs. This species is assignable to Andinobates based on the following traits: small adult size (SVL <20 mm), bright dorsal coloration, limb reticulation absent, first finger distinctively shorter than second, median lingual process absent, and maxillary and premaxillary teeth absent. Molecular phylogenetic analyses support this placement. Within Andinobates , we place A. victimatus sp. nov in the A. fulguritus group ( Brown & Twomey et al. 2011), also based on phylogenetic analyses, juvenile color pattern ( Fig. 4 View FIGURE 4 H–K; see Coloration in life), and calls of the “short buzz” type ( Brown & Twomey et al. 2011; Fig. 5 View FIGURE 5 ).
The new species can be easily distinguished from most other known members of Andinobates by its distinctive solid red dorsal coloration ( Fig. 4 View FIGURE 4 ). The only exception is the recently described A. geminisae ( Batista et al. 2014) , which has uniform orange dorsal coloration, but lacks light gray/tan pigmentation on finger and toe tips, has uniform ventral coloration, and produces calls of the “tonal buzz” type, with distinctive clicks at the beginning and end of the call, as opposed to the “short buzz” calls of A. victimatus . Moreover, calls of A. victimatus sp. nov. are shorter in duration (0.42– 0.58 s vs. 1.52– 1.6 s) and higher in peak frequency (5.65–5.86 kHz vs. 4.40–4.50 kHz; Fig. 6 View FIGURE 6 ) than those of A. geminisae . Finally, A. geminisae is, on average, smaller than A. victimatus sp. nov. (SVL 12.68 ± 0.87 mm vs. 14.23 ± 0.67 mm; t15 = 3.78, p = 0.002, N = 6 A. vic , 11 A. gem ; Fig. 6 View FIGURE 6 ). However, the SVL ranges of both species overlap slightly ( A. geminisae : 11.63–13.63 mm, A. victimatus sp. nov.: 13.22–14.98 mm). Some individuals of A. opisthomelas , A. virolinensis , and A. cassidyhornae can have nearly homogenous red dorsal coloration ( Amézquita et al. 2013; Ruiz-Carranza & Ramírez-Pinilla 1992; Silverstone 1975). However, they can be distinguished from A. victimatus sp. nov. by their ventral pattern which consists of red blotches on a black background in A. cassidyhornae , light gray spots/marbling in A. virolinensis and some populations of A. opisthomelas , and dark brown with irregular suffusions of red from the flanks in other populations of A. opisthomelas . Moreover, the three species are, on average, larger than A. victimatus ( A. cassidyhornae : 19.031 ± 0.31 mm, t11 = 17.04, p <0.0001, N = 6 A. vic , 7 A. cas ; A. virolinensis : 16.86 ± 0.91 mm, t58 = 6.85, p <0.0001, N = 6 A. vic , 54 A. vir ; A. opisthomelas : 16.80± 1.24 mm, t53 = 5.14, p <0.0001, N = 6 A. vic , 49 A. opi ; data from original descriptions and Silverstone 1975), although, again, with slight range overlap with A. virolinensis (13.22– 14.98 mm vs. 14.6–18.9 mm) and A. opisthomelas (13.22–14.98 mm vs. 14.5–19.5 mm).
In addition to coloration, A. victimatus sp. nov. can be distinguished from other lowland species of Andinobates (i.e. A. minutus and A. fulguritus groups) in the following ways ( Fig. 6 View FIGURE 6 ): The calls of A. victimatus sp. nov. are shorter than those of A. claudiae (0.42– 0.58 s vs. 0.85– 1.03 s), and have a higher peak frequency than those of A. fulguritus (5.65–5.86 kHz vs. 4.83–5.16 kHz); A. victimatus sp. nov. is smaller in body size than A. altobueyensis (13.22–14.98 mm vs. 16.30–17.00 mm).
Description of the holotype. An adult female, 13.22 mm in snout-vent length. Snout dorsally subtruncate, laterally rounded and slightly protrusive; canthus rostralis rounded; loreal region vertical and slightly concave. The head is wider than it is long, and the same width as the body (HW/GBW = 1.002); head width 29.5% of SVL; head length 25.0% of SVL; nares 1.24 times closer to tip of snout than to eyes; pupil rounded and horizontally elliptical; eye length 52.6% of head length. Tympanic ring visible, oval shaped, of moderate size (HDT 41.4% of EL); posterior and dorsal margins hidden under skin. Maxillary and premaxillary teeth absent; median lingual process absent; tongue about two times longer than wide; anterior margin of tongue not indented; posterior third of tongue not attached to floor of mouth. Hands of moderate size (HaL 26.6% of SVL); adpressed finger lengths are I<II<IV<III; adpressed toe lengths are I<II<V<III<IV; discs of fingers II, III, and IV moderately expanded; toe IV disc weakly expanded; globular, rounded subarticular tubercles; outer palmar tubercle larger than inner palmar tubercle; outer plantar tubercle smaller than inner plantar tubercle; no foot or hand webbing.
In life, the dorsum was homogeneous scarlet red, with faintly visible dorsolateral stripes; flanks were mostly uniform vermilion, with a few small, dark specks towards the ventral area. The venter was also vermilion with lesssaturated blotches transitioning from vermilion to black towards the chest and throat. Fingers and toes were light metallic gray, and palms and soles were black. The skin was slightly granular, especially in the posterior venter, cloaca, and dorsal surface of hind limbs. In ethanol (70%), the dorsum and dorsal surfaces of limbs are metallic gray; hands, feet, and articulations are tan to light brown. The venter and flanks are also metallic gray, with some scattered dark brown specks; a large dark brown blotch is present on the throat. The skin is mostly smooth, except for the ventral surface of the hind limbs and cloaca, where weak granularity is still visible.
Coloration in life ( Figs. 4, 4 View FIGURE 4 A–K). The dorsum and dorsal surfaces of limbs of all individuals in the type series are homogenous scarlet red. In some collected and uncollected individuals, a faint, complete dorsolateral stripe (type A in Grant et al. 2006) of a more saturated shade of red is visible ( Fig 4 View FIGURE 4 A-D). The scarlet red becomes less saturated in the flanks and venter, transitioning between scarlet-red, vermilion, and black in gradually changing blotches, especially in the throat, chest, and the dorsal surface of the thighs (forming a seat patch; Fig 4 View FIGURE 4 E–F). The pupil and iris are both black, and nearly indistinguishable. The nares are surrounded by thin black margins, and the anterior edge of the tympanic ring is also black, with the first quarter of the tympanum degrading from black to vermilion. Fingers and toes are light metallic gray to metallic gray, and palms and soles black to dark brown.
Juveniles reared in the laboratory ( Fig. 4 View FIGURE 4 H–K) developed tan/orange dorsolateral stripes as metamorphs (around stage 40), and later acquired a faint incomplete dorsal median stripe that extended from the tip of the snout to the posterior-dorsal region, and a labial stripe extending from just below the snout to the insertion of the arm, both reminiscent of the color pattern of some A. fulguritus . As time went by, the stripes became a more saturated red, and the dark brown background gave way to homogenous scarlet red pigmentation. Adult coloration was attained around 3.5 months after leaving the water. These individuals were reared at a lower temperature (~18–22 °C) than their natural habitat (~22–28 °C), so this process is probably quicker in nature.
Coloration in preservative (70% ethanol). In preservative, the red color turns metallic gray. Fingers, toes, and tubercles become light tan/brown color, and the pupil and cornea become light gray to white. The heterogeneity in ventral coloration becomes more defined in preservative, as the black blotches become dark brown, or stay black, while the scarlet red/vermilion background becomes metallic gray.
Vocalization ( Figs. 5–6 View FIGURE 5 View FIGURE 6 ). Similar to other members of the A. fulguritus group, A. victimatus sp. nov. produces calls of the “short buzz” type, consisting of a series of pulses modulated in amplitude but not frequency ( Brown & Twomey et al. 2011; Myers & Daly 1976). Calls range from 0.42– 0.58 s in length (mean = 0.49 ± SD = 0.06 s), and contain 99–190 pulses per call (142 ± 42.6). The peak frequency ranges between 5.65–5.86 kHz (5.75 ± 0.08 kHz), and the frequency interquartile bandwidth between 0.76–1.03 kHz (0.88 ± 0.11 kHz).
Natural history. A. victimatus sp. nov. is a terrestrial species, which inhabits the understory of lowland forests in the Urabá region of Northwestern Colombia ( Fig. 7 View FIGURE 7 ). Males call to defend territories and attract mates, and were typically observed doing so from the leaf litter, or perched on low branches (<1 m above the ground) and fallen logs. A few males were also heard calling from bromeliads up to ~ 3 m above the ground. Call activity peaked between 7:30 and 9:30 AM, with occasional calling throughout the rest of the day.
Egg clutches are presumably laid within male territories, and tadpoles are transported by males, one at a time, to bromeliad tanks. Tadpoles were observed in bromeliads up to 4m above the ground (although we did not survey plants above this height). All of the observed tadpoles were in tanks of Guzmania musaica bromeliads ( Fig. 7 View FIGURE 7 D), which leads us to speculate that A. victimatus sp. nov. prefers this bromeliad over other similarly-sized species (e.g. other Guzmania or Tillandsia ), for yet unknown reasons. At the type locality, A. victimatus sp. nov. is sympatric with Oophaga histrionica ( Anura : Dendrobatidae ), which deposits larvae in similar phytotelmata. However, we did not find any O. histrionica tadpoles in G. musaica tanks; only in smaller bromeliad species. Whether this indicates some instance of resource partitioning, competition, or another kind of ecological interaction between the two species remains to be studied.
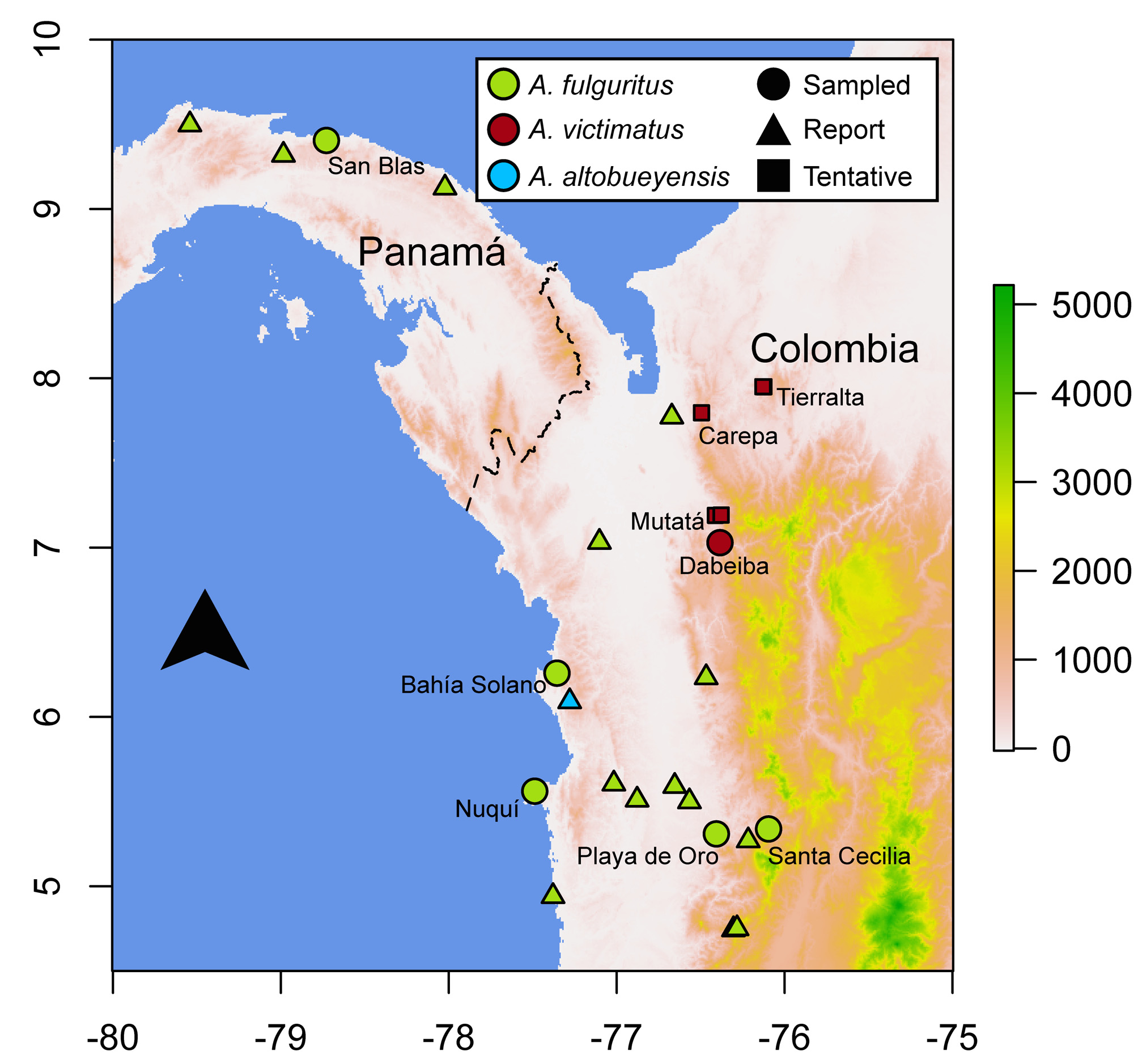
Distribution and conservation status. This species occurs in the Urabá region of Northwestern Colombia, inhabiting tropical rain forests at ~ 200– 600 m.a.s.l. ( Figs. 1 View FIGURE 1 , 7 View FIGURE 7 ) in the foothills of the Western Andes, west of the Paramillo Massif, on the Atrato River drainage. The type locality is in the Llorona Canyon in Dabeiba, Antioquia, Colombia, just southwest of the Massif. We have become aware of reports of similar frogs from other biologists and conservationists working in the Urabá region (Carlos Bran-Castrillón, pers. com.), which would extend the new species’ distribution north into the municipalities of Mutatá and Carepa, almost to the Gulf of Urabá, on the Caribbean coast ( Fig. 1 View FIGURE 1 ). In addition, two specimens at the Natural History Museum of the Universidad de Antioquia (MHUA-A 3382 and 3864), currently assigned to A. opisthomelas , are from the municipality of Tierralta, Córdoba, at ~ 590 m. a.s.l ( Fig. 1 View FIGURE 1 ). It is possible that these two specimens actually correspond to A. victimatus sp. nov., since A. opisthomelas does not normally occur below 1100 m.a.s.l ( Silverstone, 1975), which would mean that this species is distributed around the northern end of the Paramillo Massif, into the Sinú River drainage. As we have not examined specimens, alive or preserved, from any of these localities, we consider them tentative reports.
Andinobates victimatus sp. nov. has, to the best of our knowledge, not been reported south or west of the type locality. However, given the continuous rain forests and gentle topography of the Northeastern Chocó, it is likely that this species does extend into these areas. Nonetheless, Silverstone did not find it when collecting A. fulguritus in the Arquía river, ~ 85 km south of the type locality, in the 1960s, and it has not been reported in the vicinities of Quibdó (~ 130 km south) or the San Juan River drainage (> 180 km south), both well sampled in terms of Dendrobatid frogs. Therefore, we suspect that this species’ range does not extend too far south of the type locality. Further exploration and inspection of museum specimens are needed to get a better grasp of this species’ geographic distribution.
In spite of the current uncertainty regarding the distribution of A. victimatus sp. nov., it seems clear that its extent of occurrence is less than 5,000 km 2. It is currently known from one locality with certainty, plus five tentative additional ones, and the forests in this species’ range have been, and will probably continue to be under strong deforestation pressures due to mining (both legal and illegal), cattle farming, and large-scale agriculture (e.g. banana and plantain plantations), which reduce the amount and quality of available habitat, and increase its fragmentation. Therefore, we tentatively designate A. victimatus sp. nov. as Endangered (EN: B1a, biii; IUCN, 2001). However, it is possible that the range of A. victimatus sp. nov. does exceed 5,000 km 2, in which case this species should be listed as Vulnerable (VU; B1a, biii). Most of the range of A. victimatus sp. nov. surrounds the Nudo de Paramillo National Natural Park, a 460,000 ha nature reserve, managed by the Colombian government, which is closed to the public. However, we are unaware of any reports of A. victimatus sp. nov within the park, which encompasses most of the Paramillo Massif above 700 m. a.s.l. This national park, nonetheless, protects ~250,000 ha in the 700–1000 m.a.s.l elevation belt, where A. victimatus could exist at the higher end of its elevational range. Considerably more research on this species’ distribution, habitat requirements, and population ecology is needed to confidently assign it to a category of threat.
Voucher Species Locality Study GenBank Accession
16S COI Cytb ……continued on the next page
Voucher Species Locality Study GenBank Accession
16S COI Cytb GECOH 1216 Andinobates opisthomelas I la đel SƟl, Guatapé, AntiƟquia, Amézquita et al. JQ936634 View Materials KY885051 View Materials JQ936620 View Materials
CƟlƟmbia 2013
GECOH 297 Andnobates bombetes Bosque YƟtƟcƟ Natural Re erve, YƟtƟcƟ, Thi tuđy KY885045 View Materials KY885067 View Materials KY885092 View Materials
Valle đel Cauca, CƟlƟmbia
GECOH 305 Andinobates virolinensis CƟ tilla đe fara, VirƟlín, Santanđer, ƂrƟwn et al. 2011; JN635873 View Materials KY885068 View Materials JQ936626 View Materials
CƟlƟmbia Amézquita et al.
2013
GECOH 474 Andinobates tolimensis Falan , TƟlima, CƟlƟmbia ƂrƟwn et al. 2011; JN635857 View Materials KY885069 View Materials JQ936632 View Materials
Amézquita et al.
2013
GECOH 1523 Andinobates cassidyhornae La Me enia, Jarđín, AntiƟquia, CƟlƟmbia Amézquita et al. JQ936636 View Materials KY885054 View Materials JQ936622 View Materials
2013
MVUP 2428 Andinobates geminisae 6 Km ea t Ɵf El Carmen (RíƟ Ƃelén), Ƃati ta et al. 2014 KM212167 View Materials KM212166 View Materials - Heađwater Ɵf RíƟ CanƟ, DƟnƟ Ɵ, CƟlón,
Panamá
USNM-FS Andinobates claudiae SƟuth enđ Ɵf I la PƟpa, 1km E Ɵf FrƟ t et al. 2004; DQ283042 View Materials DQ502748 View Materials DQ502453 View Materials
59980 SunwƟƟđ Channel, ƂƟca đel TƟrƟ, Grant et al. 2006
Panamá
Paratype; ** HƟlƟtype
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.