Steinernema pui, Qiu, Lihong, Zhao, Jingxiu, Wu, Zhongdao, Lv, Zhiyue & Pang, Yi, 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.206861 |
|
DOI |
https://doi.org/10.5281/zenodo.6193913 |
|
persistent identifier |
https://treatment.plazi.org/id/038DF34E-7E0C-FFE6-FF74-33E1FB71FA7B |
|
treatment provided by |
Plazi |
|
scientific name |
Steinernema pui |
| status |
sp. nov. |
Steinernema pui sp. n. description
Measurements. Morphometrics of the holotype (the first generation male), allotype (the first generation female) and paratypes of IJs and the first- and second- generation males and females are listed in Table 1 View TABLE 1 .
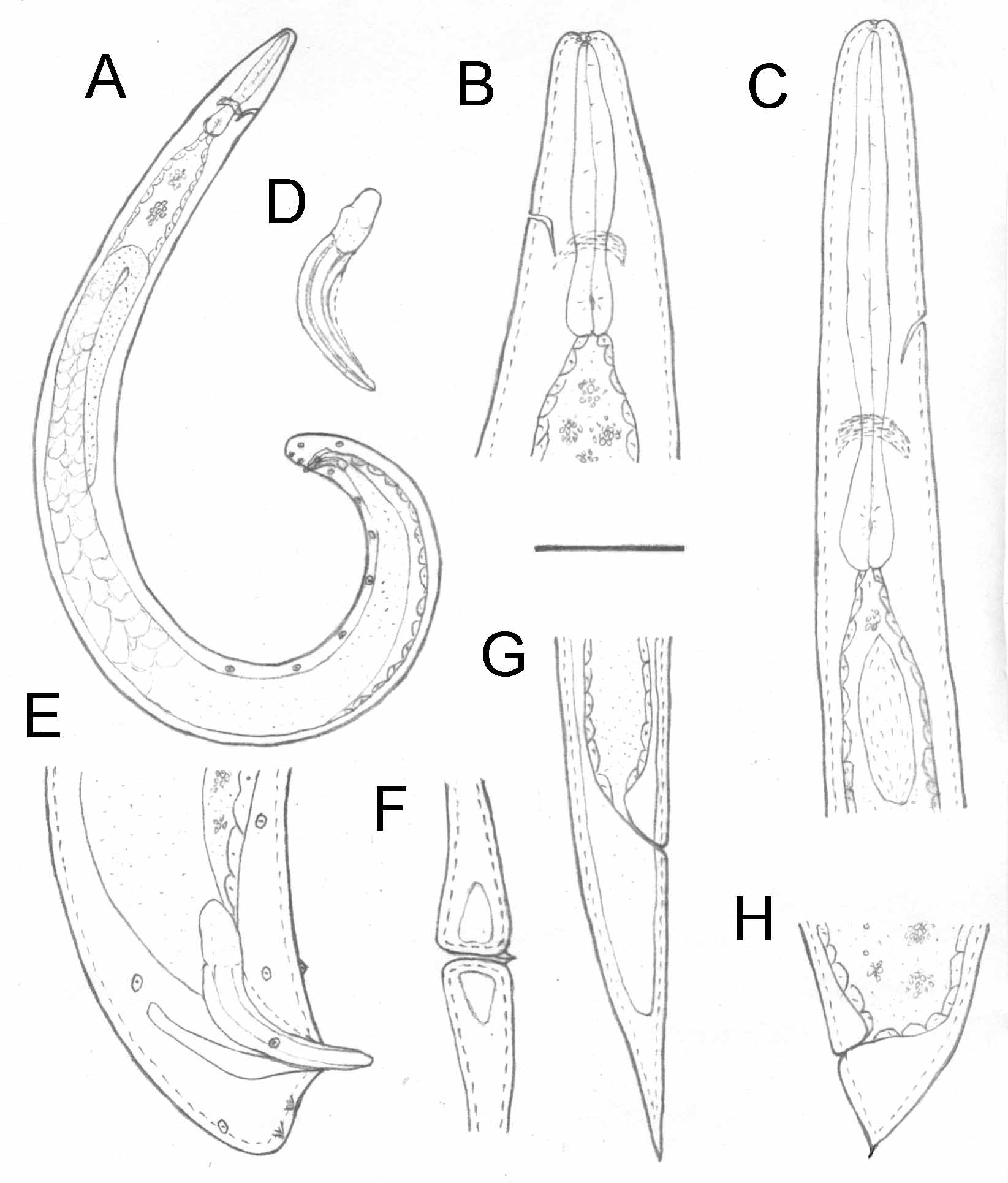
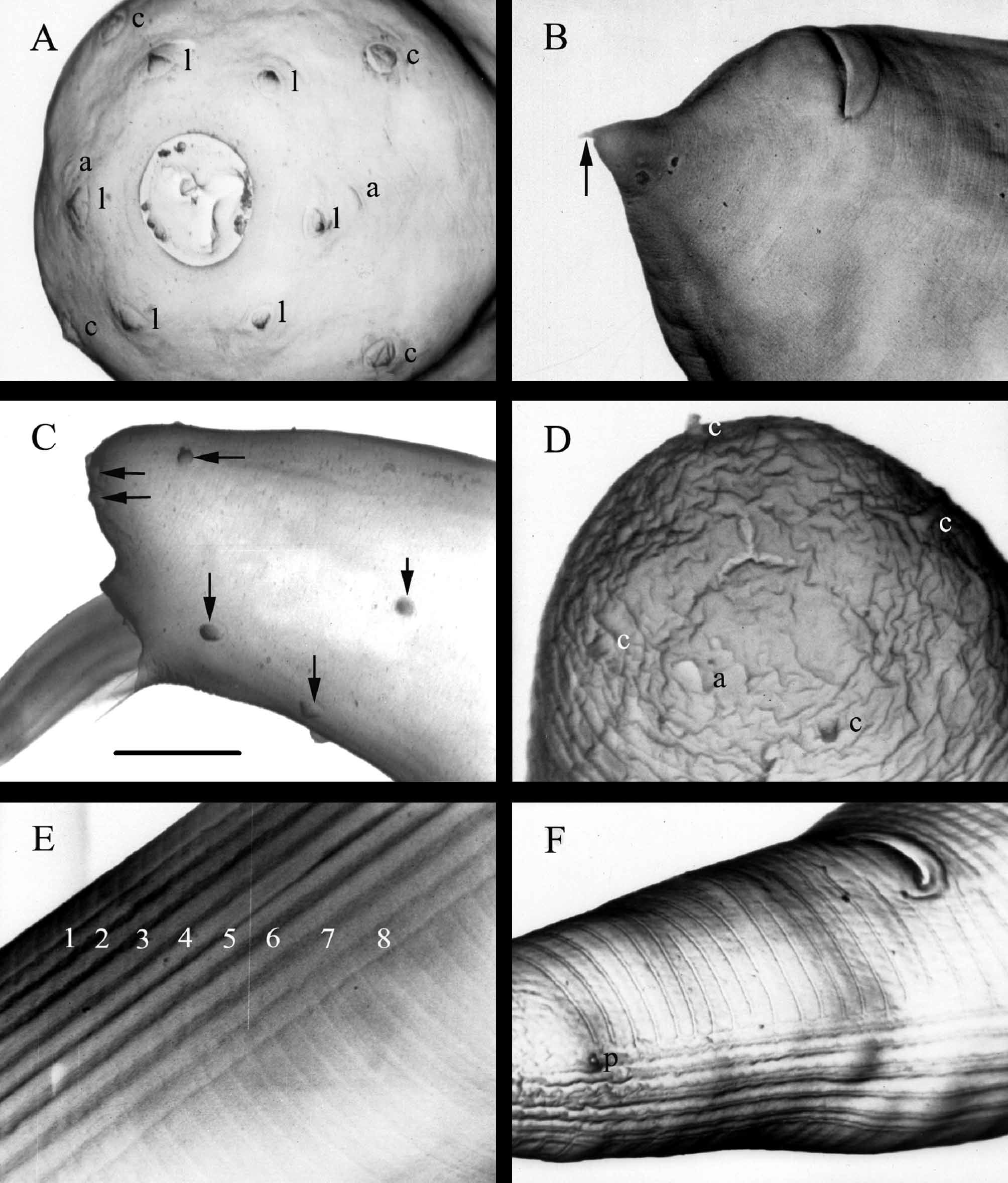
First-generation males: Body of heat-relaxed specimens C-shaped and curved posteriorly. Head truncated and slightly swollen anteriorly. Six lips fused at base, each with a papilla. Four cephalic papillae present. Two amphidial apertures distinct, located behind lateral labial papillae ( Fig. 2 View FIGURE 2 A). Stoma shallow, circular anteriorly and triradiate internally. Pharynx extends nearly to mouth opening. Both cheilorhabdions and prorhabdions small but distinct.
Pharynx muscular, with a cylindrical procorpus, metacorpus somewhat swollen, isthmus well defined, basal bulb slightly enlarged. The maximum diameter of metacorpus is equal to that of basal bulb. Nerve ring surrounds anterior portion of basal bulb. The excretory pore opening located slightly anterior to nerve ring. Lateral fields and phasmids not observed. Gonad single, reflexed. Spicules paired with a light brown color, moderately curved. The spicule length/width of the mature male is about 5.2 ( Fig. 3 View FIGURE 3 A and B). Spicule head longer than wide with length/ width ratio of about 1.7 (1.5–2.0). Lateral and dorsal limb distinct, starting from head and extending to the spicule tip. Ventral limb starts from head and end well before the spicule tip. The tip of spicule blunt, bearing an aperture located on the tip with a circular shape and a slit-like concave located on the ventral side of the blade closed to the tip ( Fig. 3 View FIGURE 3 D). This character was observed clearly in all of 4 specimen in posterior and ventral view (the only position that enables this character to be observed) among about 20 dissected spicules examined under SEM. Velum moderately developed, starting from anterior portion of ventral limb and ending at the tip of the ventral limb. Gubernaculum is boat-shaped in lateral view. In ventral view, it tapers anteriorly and ends at a slightly enlarged, ventrally bent head ( Fig. 3 View FIGURE 3 E and F). Cuneus is needle-shaped and short. Spicules of the young males ( Fig. 3 View FIGURE 3 C) are slightly different from those of the mature ones ( Fig. 3 View FIGURE 3 A and B). Twelve pairs and a single precloacal genital papillae distributed in a pattern showed in Fig. 1 View FIGURE 1 A and E and Fig. 2 View FIGURE 2 C, including seven pairs preanal papillae, one pair adanal, one pair dorsolateral and three pair postanal (two being subterminal and one subdorsal). Tail conoid and short, without mucron.
Character1 First generation Second generation Infective 1EP, NR and ES: distance from anterior end to excretory pore, nerve ring and the base of pharynx, respectively; H%: hyaline tail length in % of total tail length. 2Mean ± SD and range in µm.
Second-generation males: Similar to that of the first generation male except that the excretory pore is located much more posterior and most morphometrics, such as spicule and gubernaculum length, are smaller. Tail conoid without mucron.
First generation female: Body C-shaped when killed by gentle heat. Cuticle smooth or with faint annules. Head rounded and slightly truncated anteriorly ( Fig. 1 View FIGURE 1 B). The morphology of the anterior part and pharynx is similar to those of males. The excretory pore opening is anterior to nerve ring. Lateral fields and phasmids not observed. Gonads didelphic, reflexed. Vagina muscular and short. Vulva a transverse slit, slightly protruding from body surface and with a short double flapped epiptygma. Tail conoid, pointed, with a short projection ( Fig. 1 View FIGURE 1 G; Fig. 2 View FIGURE 2 B).
Second-generation female: Similar to the first generation female but smaller. Vulva asymmetric, protruding from body surface. Short double flapped epiptygma present in the first generation female indistinct or absent in the second generation females. Tail longer than anal body width, tapering to a pointed end.
Infective juveniles: Body slender. Mouth and anus closed. Anterior end rounded, slightly truncated and continuous with body contour ( Fig. 1 View FIGURE 1 C, Fig. 2 View FIGURE 2 D). Labial region smooth, papillae not seen. Amphidial apertures distinct, slit –shaped. Four cephalic papillae prominent. Pharynx long and narrow, isthmus distinct and surrounded by nerve ring, basal bulb slightly elongated, cardia indistinct ( Fig. 1 View FIGURE 1 C). Excretory pore located anterior to nerve ring, in slightly posterior position of pharynx. Bacterial pouch located in the anterior portion of the intestine. Cuticle with prominent transverse striations. Lateral field begins anteriorly with one line and increases to 9 posterior to the base of pharynx, making a total of 8 ridges ( Fig. 2 View FIGURE 2 E). The nine-line pattern extends posteriorly to a position closed to anus where the number of ridges reduced to five followed by two and then disappeared ( Fig. 2 View FIGURE 2 F). Phasmid distinct, located on the posterior end of the first ridge from ventral side. Tail short, attenuate and tapering evenly ( Fig. 1 View FIGURE 1 F). Hyaline portion occupying 46% of the tail length.
Type locality and host. The soil sample was collected from a rubber plantation at Xiao-jie town, Jing-hong city, Xi-shuang-ban-na district (22.01o N, 100.47 o E), Yunnan province, People’s Republic of China. The type host of this nematode in nature is unknown as it was recovered from soil using Galleria larvae as bait.
Type specimens and etymology. The slides of holotype (first generation male), allotype (first generation female) and paratypes of about 60 infective juveniles on three slides, 10 each of the first and second generation males and females on 20 slides (one male and one female from the same generation on each slide) of S. pui sp. n. were deposited in the State Key Lab for Biocontrol ( SKLB), School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China. Additional paratypes (one each of the first and second generation male and female and about 20 IJs on three slides) will be deposited in the Nematode Collection of the Department of Agriculture, USA. Living infective juveniles are also preserved in liquid nitrogen in the nematode collection of SKLB, Zhongshan University.
This species is named after the late Professor Zhelong Pu, a distinguished scientist in the area of biological control and the founder and first director of SKLB, Sun Yat-sen University.
Cross-breeding. Normal offspring were observed on the majority of slides on which two inoculated IJs developed into opposite sex adults in the self-cross controls of CWL05, GDc339 and YNd339. However, no offspring were observed from any slide in the cross-breeding treatments of YNd339 x CWL05 and YNd339 x GDc339.
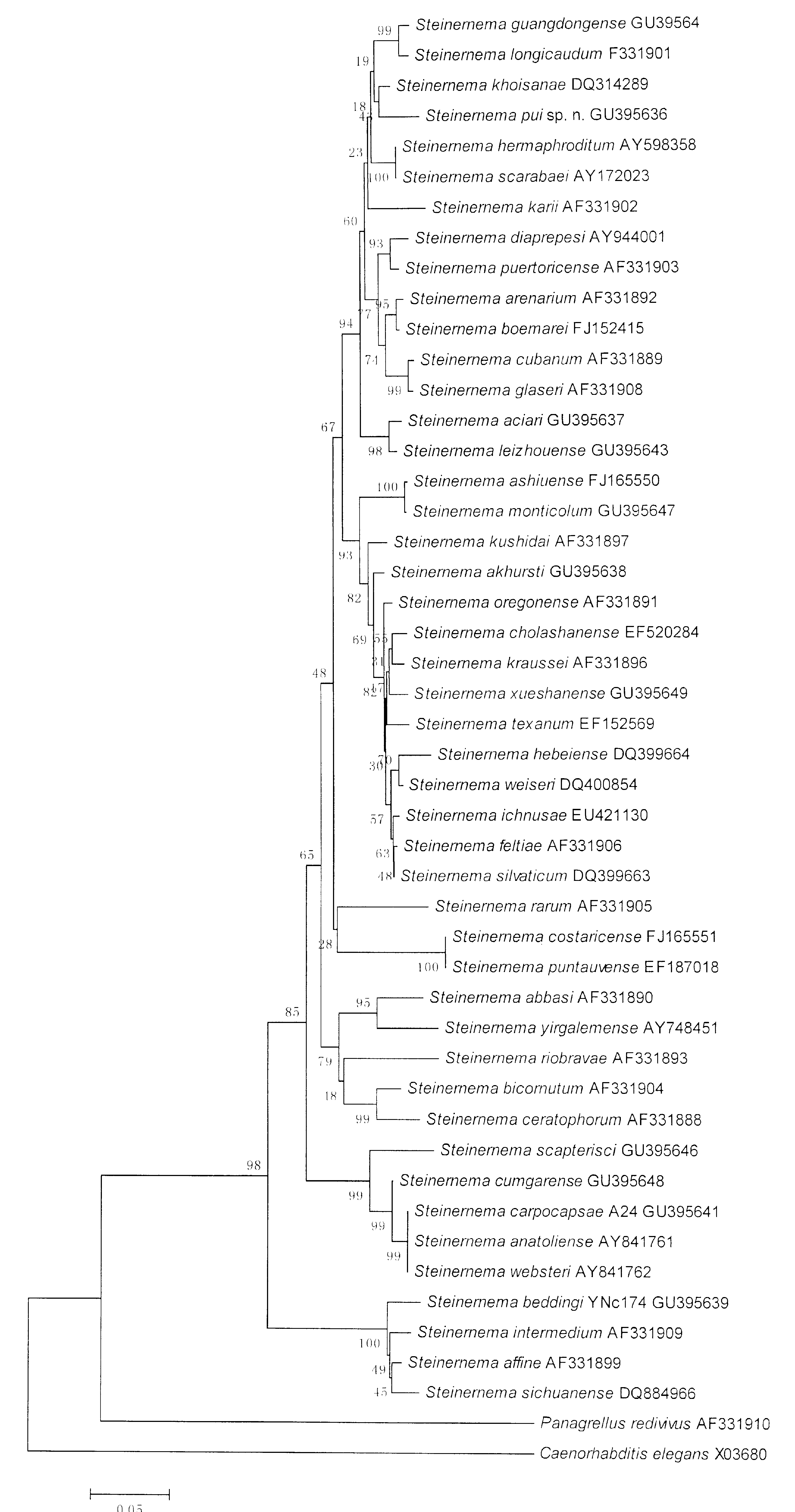
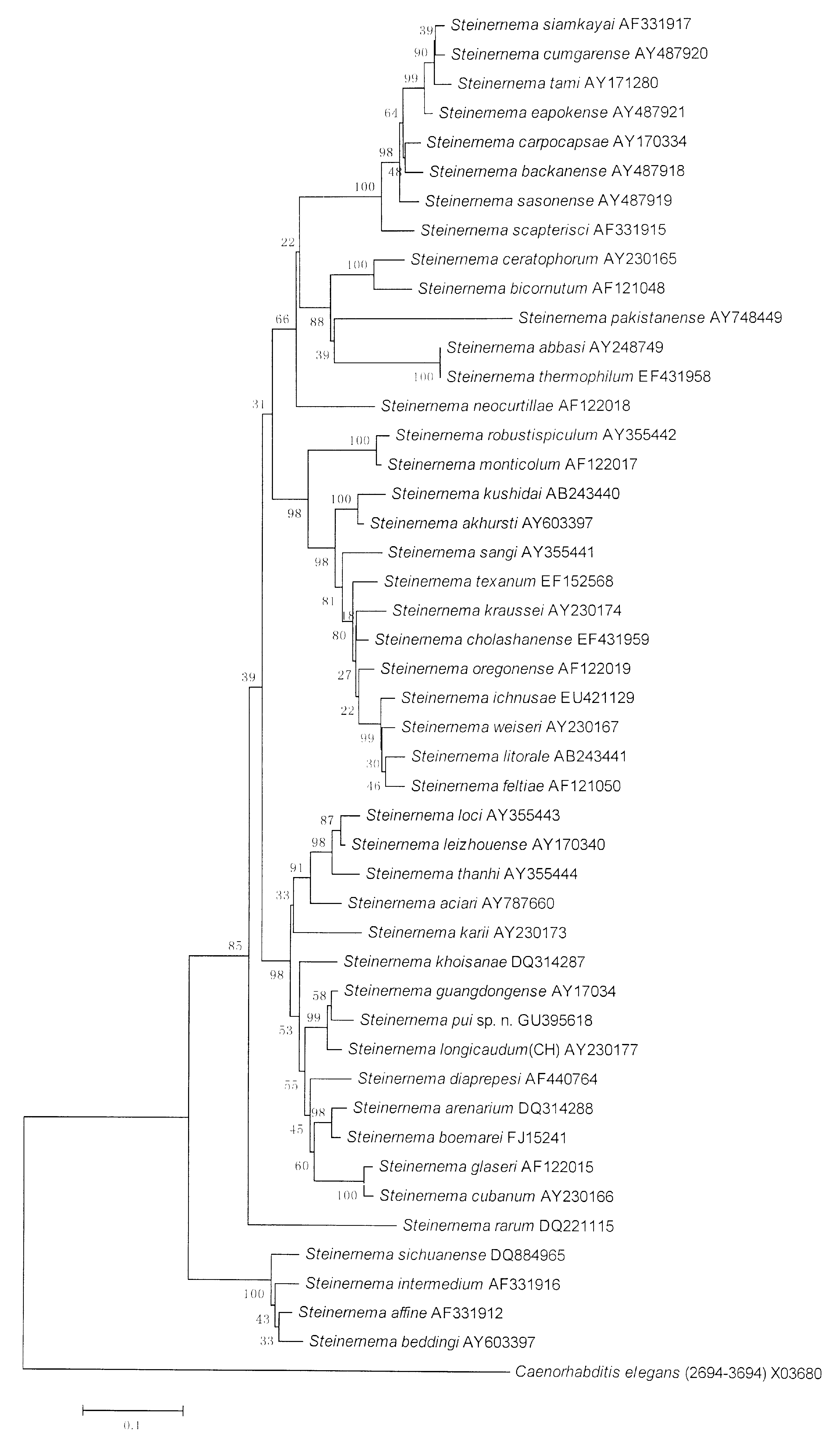
Molecular characterization. The lengths of ITS and partial 28S rDNA of S. pui sp. n. amplified using the above mentioned primers were 977bp (235A, 255G, 291T and 195C) and 800bp (193A, 247G, 196T and 164C), respectively. The phylogenetic tree of the partial 28S rDNA ( Fig. 4 View FIGURE 4 ) shows that S. pui sp. n. forms a monophyletic group with S. khoisanae , S. longicaudum , S. guangdongense , S. scarabaei and S. hermaphroditum . This is a subgroup of the S. glaseri group that comprises EPNs with long IJ body and adopted a cruiser host finding strategy ( Campbell et al., 2003). The sequence alignment of the S. pui sp. n. group ( Fig. 5 View FIGURE 5 ) shows that it has 22 diagnostic characters (= number of base pairs, in the same column of the alignment, present in one sequence but not in others) in the group and differs from its closest nematode, S. khoisanae by 33 characters while there are only 11 different characters between S. guangdongense and S. longicaudum . Pairwise distances ( Table 2 View TABLE 2 ) clearly differentiate it from all other nematodes in the group. The phylogenetic tree of the rDNA ITS regions ( Fig. 6 View FIGURE 6 ) support the relationship of S. pui sp. n. with other Steinernema species revealed by the partial 28S rDNA sequences. The pairwise distances of the ITS sequences ( Table 3 View TABLE 3 ) can also be used to differentiate the new species from other nematodes in the group.
Diagnosis. S. pui sp. n. is characterized by its unique nucleotide sequences of either the partial 28S rDNA or the rDNA ITS regions. Morphologically, it is characterized by the combination of the features of various developmental stages of the nematode. For IJs, the combination of the following characters: body length (1004 ± 75 μm); distance from anterior end to excretory pore (85 ± 4 μm), to the base of pharynx (114 ± 8 μm); tail length (69 ± 5 μm); E% (77 ± 4.5); lateral field with eight evenly distributed and identical ridges at the middle body portion; and tail short and attenuate with a hyaline portion occupying 40–50% of the tail length can be used to differentiate the new species from other Steinernema species. For the first generation males, the new species can be recognized by spicule length (84 ± 4 μm, measured along the arch); GS (0.74 ± 0.04); SW (1.52 ± 0.10); D (77 ± 5.8); spicule possesses an aperture on the tip and a pit on the ventral side of the lamina close to the tip; gubernaculum with a short needle-shaped cuneus; tail conoid and short without mucron. For female, S. pui sp. n. is recognized by a conoid and pointed tail with a short mucron on the tip and a slightly protruding and symmetrical vulva with a short double flapped epiptygma.
Relationships. Both molecular ( Figs. 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 ) and morphological characters ( Table 1 View TABLE 1 and 4 View TABLE 4 ) showed congruently that S. pui sp. n. belongs to the S. glaseri group. 28S rDNA sequence shows that the new species is most closely related to S. khoisanae . However, the genetic distance between them is 3.2 ( Table 2 View TABLE 2 ), greater than that of many closely related described species, such as S. cubanum and S. glaseri (0.9), S. longicaudum and S. guangdongense (1.4) and S. boemarei and S. arenarium (2.7). Several characters of various developmental stages can also separate the new species from S. khoisanae : IJ tail length of S. pui sp. n. is shorter (69 ± 5) vs (85 ± 5), the distance from anterior end to excretory pore is smaller (85 ± 4) vs (95 ± 5) and diameter greater (36 ± 2) vs (31 ± 2); vulva of the first generation females of S. pui sp. n. has a short double flapped epiptygma vs absence in S. khoisanae ; SW (the ratio of spicule length to tail width) of the first generation male of the new species is 1.52 ± 0.1, lower than that of S. khoisanae (2.03 ± 0.5); an aperture were seen on the spicules tip of the first generation S. pui sp. n. but this character is not reported in S. khoisanae . S. pui sp. n. can be distinguished from the rest of Steinernema species of the S. glaseri group, including S. khoisanae , S. guangdongense , S. longicaudum , S. scarabaei and S. karii by morphological characteristics of the IJs and the first generation males as listed in Table 4 View TABLE 4 .
TABLE 1. Morphometrics of Steinernema pui sp. n.
| Holotype | Male | Allotype | Female | Male | Female | Juvenile | |
|---|---|---|---|---|---|---|---|
| n L Greatest body diam EP | 2025 130 140 | 20 2059±124 2 (1800–2350) 137±15 (118–180) 152±13 | 5200 238 133 | 20 6081±1151 (4850–8875) 228±32 (155–275) 147±17 | 10 1425±67 (1300–1500) 84±7 (70–90) 138±10 | 10 2495±278 (2070–2950) 138±10 (125–150) 126±6.1 | 20 1004±75 (900–1120) 36±2 (33–40) 85±4 |
| NR ES Tail length (T) Anal body diam. (ABD) Spicule length (SP) Gubernaculum | 150 193 34 55 80 65 | (130–180) 157±10 (143–188) 196±12 (175–228) 32 ± 2 (29–38) 55±3 (48–63) 84±4 (78–88) 62±2 | 150 225 55 90 | (125–188) 166±17 (150–218) 236±21 (213–288) 57±10 (43–75) 98±13 (85–125) | (120–150) 110±12 (93–125) 141±13 (120–162) 27±3.7 (21–30) 38±4 (35–48) 65±4.3 (58–70) 44±4.4 | (120–137) 137±9.3 (125–152) 183±16 (162–207) 60±3.7 (53–63) 56±5 (50–65) | 80–95 117±7 (100–125) 144±8 (130–150) 69±5 (60–80) 22±1 (20–25) |
| length (GU) a b c | 15 10 60 | (58–65) 15±1.3 (14 –16) 11± 1.4 (9.5–12) 64±6 | 22 23 94 | 27±5 (20–35) 26±3 (22–31) 107± 14 | (35–50) 17±3 (14–20) 10.1±0.2 (9.6–11) 53±4 | 18± 4 (14–22) 14±3 (11–18) 42±3 | 28±1.7 (26–31) 7.03±0.74 (6.00–8.15) 15±0.8 |
| c’ H% D% =EP/ES x 100 | 0.62 73 | (58–70) 0.58±0.06 (0.53–0.61) 77±5.8 | 0.61 59 | (96–118) 0.58±0.07 (0.53–0.61) 62.3±4.5 | (48–66) 0.71±0.06 (0.53–0.61) 98±12 | (37–46) 1.07±0.1 (0.53–0.61) 68.9±6.7 | (13–16) 3.1±0.2 (2.9–3.3) 45±3.7 (40–50) 59.5±3.04 |
| E% = EP/T x 100 SW=SP/ABD | 412 1.45 | (70–91) 465±48 (403–512) 1.52±0.10 | 241 | (54–71) 258±35 (201–312) | (77–114) 511±48 (389–621) 1.71±0.20 | (57–81) 210±45 (139–272) | (55.00–66.04) 125±8.0 (109–142) |
| GS=GU/SP | 0.81 | (1.40–1.84) 0.74±0.04 | (1.32–1.93) 0.68±0.08 | ||||
| V | (0.69–0.81) | 51 | 50±5 (37–55) | (0.52–0.78) | 52±2.3 (50–57) |
TABLE 2. Percentage of similarity (upper triangle) and genetic distance (lower triangle) on the sequences of D 2 D 3 domain of 28 S rDNA of Steinernema pui sp. n. with other closely related Steinernema species.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. S. boemarei | *** | 95.0 | 92.1 | 89.2 | 92.4 | 93.1 | 92.4 | 94.0 | 92.7 | 90.2 | 90.7 | 90.8 | 89.4 |
| 2. S. arenarium | 2.7 | *** | 91.7 | 89.4 | 93.0 | 92.2 | 92.6 | 91.9 | 91.4 | 90.1 | 90.0 | 90.2 | 89.9 |
| 3. S. glaseri | 6.0 | 5.2 | *** | 97.1 | 92.1 | 92.0 | 92.0 | 90.5 | 90.9 | 88.2 | 88.9 | 89.9 | 88.0 |
| 4. S. cubanum | 6.6 | 5.7 | 0.9 | *** | 91.7 | 91.0 | 91.1 | 89.1 | 89.7 | 87.5 | 88.3 | 89.0 | 87.6 |
| 5. S. puertoricense | 5.1 | 4.0 | 6.3 | 6.4 | *** | 97.6 | 95.9 | 94.2 | 95.7 | 91.5 | 93.6 | 94.7 | 91.9 |
| 6. S. diaprepesi | 5.0 | 4.7 | 6.4 | 6.9 | 2.2 | *** | 96.2 | 94.7 | 96.2 | 92.9 | 93.6 | 94.6 | 91.0 |
| 7. S. khoisanae | 4.8 | 4.8 | 6.3 | 6.7 | 3.2 | 2.8 | *** | 95.5 | 96.1 | 95.4 | 94.4 | 95.4 | 93.5 |
| 8. S. longicaudum | 4.9 | 5.4 | 7.8 | 8.3 | 5.1 | 4.7 | 3.9 | *** | 98.0 | 94.2 | 94.0 | 95.2 | 92.2 |
| 9. S. guangdongense | 5.5 | 5.8 | 7.6 | 8.0 | 4.1 | 3.7 | 3.2 | 1.4 | *** | 94.5 | 94.5 | 95.7 | 93.0 |
| 10. S. pui sp. n. | 6.9 | 7.4 | 9.7 | 10.1 | 7.0 | 6.2 | 4.5 | 4.7 | 4.7 | *** | 92.8 | 93.6 | 92.1 |
| 11. S. hermaphroditum | 5.9 | 5.9 | 8.0 | 8.4 | 4.7 | 4.4 | 3.4 | 3.5 | 3.9 | 4.9 | *** | 99.2 | 91.6 |
| 12. S. scarabaei | 6.1 | 5.9 | 7.9 | 8.3 | 4.6 | 4.4 | 3.3 | 3.4 | 3.6 | 4.8 | 0.0 | *** | 91.8 |
| 13. S. karii | 7.6 | 7.8 | 9.4 | 9.5 | 6.7 | 7.2 | 5.8 | 6.8 | 6.2 | 7.2 | 5.7 | 6.5 | *** |
TABLE 3. Percentage of similarity (upper triangle) and genetic distance (lower triangle) on the rDNA internal transcribed spacer region sequences of Steinernema pui sp. n. with other closely related Steinernema species.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. S. loci *** | 82.7 | 58.8 | 62.5 | 61.3 | 47.9 | 50.2 | 52.1 | 56.9 | 58.5 | 54.1 | 55.0 | 58.7 | 55.0 |
| 2. S. leizhouense 4.3 | *** | 63.8 | 63.8 | 54.3 | 52.3 | 59.3 | 55.0 | 53.9 | 58.2 | 56.3 | 59.8 | 64.6 | 54.2 |
| 3. S. thanhi 8.3 | 6.6 | *** | 52.5 | 52.8 | 45.8 | 44.0 | 49.3 | 52.5 | 47.6 | 50.1 | 50.4 | 51.5 | 47.8 |
| 4. S. aciari 19.9 | 18.7 | 18.7 | *** | 63.8 | 62.3 | 61.0 | 60.3 | 62.7 | 65.3 | 64.0 | 61.0 | 62.7 | 58.0 |
| 5. S. diaprepesi 20.3 | 19.1 | 21.1 | 18.3 | *** | 57.0 | 63.1 | 62.0 | 58.1 | 59.2 | 68.9 | 55.4 | 58.9 | 61.6 |
| 6. S. glaseri 30.0 | 28.3 | 26.9 | 25.0 | 25.2 | *** | 89.3 | 59.1 | 65.5 | 56.2 | 60.4 | 63.4 | 61.5 | 59.3 |
| 7. S. cubanum 29.5 | 28.4 | 27.5 | 27.4 | 27.5 | 4.4 | *** | 60.1 | 63.3 | 61.8 | 61.0 | 62.2 | 61.8 | 59.0 |
| 8. S. boemarei 31.1 | 29.0 | 28.0 | 28.9 | 29.7 | 18.2 | 19.5 | *** | 68.1 | 52.3 | 56.4 | 53.9 | 60.5 | 54.4 |
| 9. S. arenarium 28.1 | 26.0 | 24.2 | 27.3 | 25.8 | 16.6 | 17.3 | 12.6 | *** | 59.2 | 60.2 | 58.2 | 63.7 | 58.3 |
| 10. S. longicaudum 24.5 | 23.0 | 21.6 | 21.1 | 17.5 | 22.6 | 24.1 | 25.6 | 22.0 | *** | 84.0 | 77.2 | 67.1 | 65.2 |
| 11. S. guangdongense 23.7 | 22.9 | 21.5 | 21.5 | 15.7 | 22.3 | 24.0 | 23.9 | 21.6 | 4.3 | *** | 80.0 | 65.3 | 63.5 |
| 12. S. pui sp. n. 23.5 | 22.3 | 21.9 | 23.3 | 18.0 | 22.1 | 24.5 | 23.9 | 21.8 | 8.9 | 7.2 | *** | 63.2 | 62.9 |
| 13. S. khoisanae 27.5 | 27.1 | 27.6 | 26.0 | 22.3 | 29.5 | 31.1 | 27.5 | 26.4 | 17.5 | 15.4 | 17.3 | *** | 60.4 |
| 14. S. karii 26.9 | 27.5 | 25.1 | 22.9 | 20.4 | 28.6 | 29.1 | 27.1 | 25.6 | 19.8 | 17.8 | 19.0 | 23.0 | *** |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.