Teratozephyrus elatus, Hsu & Lu, 2005
|
publication ID |
https://doi.org/ 10.1080/00222930410001708623 |
|
persistent identifier |
https://treatment.plazi.org/id/038E2A2C-184B-FFD2-0DB2-F72469CFF9DC |
|
treatment provided by |
Carolina |
|
scientific name |
Teratozephyrus elatus |
| status |
sp. nov. |
Teratozephyrus elatus n. sp.
( Figures 2–5 View Figures 2–9 , 10–16 View Figures 10–13 View Figures 14–19 , 20 View Figures 20, 21 )
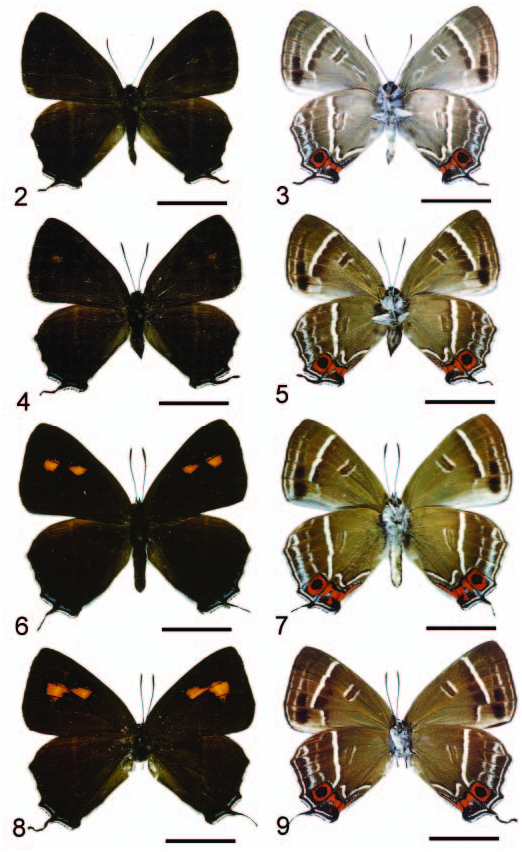
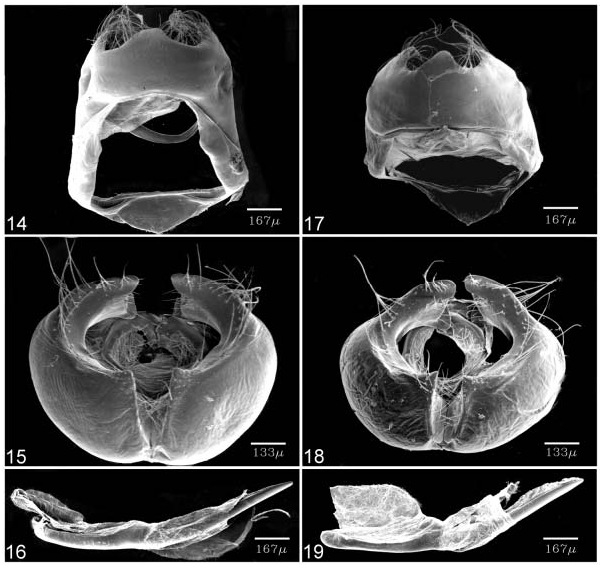
Description. Male ( Figures 2, 3 View Figures 2–9 ): FL 15.2–17.3 mm (mean 16.2¡ 0.8 mm, n55); AL 7.3– 8.2 mm (mean 7.8¡ 0.4 mm, n55). Head: hairy, vertex, frons dark brown but with white mesad; a white, narrow rim surrounding eye; eye semi-oval, densely covered with long, buff setae; labial palpus porrect, with third segment pointed downwards and much shorter than second segment, covered with white scaling mottled with black; scales on venter slender, long and hair-like; maxillary palpus reduced, invisible; proboscis unscaled, pale buff in colour; antenna smoothly scaled, naked at terminal end of nudum and along inner surface distad where short trichoid sensilla present. A pair of white dots at base of most flagellomeres, but attenuate toward nudum. Thorax: buff dorsad, white ventrad; tegulae covered by buff tinged with red hairs; legs covered with white scales, mottled with brown. Fore wing: termen, costa slightly concave, dorsum nearly straight. Ground colour of upperside uniformly dark brown, with underside markings barely visible by transparency. Ground colour of underside grey. Discal spot forming brown bar edged with white. Distal band of central symmetry system represented as tilted, uneven white line edged with prominent brown band proximally, running from costa toward Cu 2; white scales with tendency to extend basad, intersecting brown band. Submarginal band and ‘‘g’’-element as defined by Nijhout (1991) fused into prominent, dark brown band edged with white, attenuate toward apex. Fringe with white cilia. Hind wing: contour of wing slightly produced at distal end of M 1, M 3 and Cu 1; Cu 2 bearing long, ‘‘tail’’-like projection distad (length 4.6¡ 0.3 mm, n55). Ground colour of upperside uniformly dark brown, overlaid with metallic blue scaling distally in cell Cu 1 and Cu 2, with underside markings visible by transparency. Ground colour of underside grey. Discal spot forming brown bar edged with white. Distal band of central symmetry system forming prominent white line edged with brown proximally, nearly straight but uneven, from dorsum to vein Cu 2, re-bent three times in cell Cu 2, forming a prominent ‘‘W’’-shaped band. Proximal band of central symmetry system obsolete. Submarginal band consisting of faint, broad white band, a black, round dot enclosed within orange circle in cell Cu 1, and a tornal orange patch in cell Cu 2, both edged by black scales basad. ‘‘g’’-Element forming a faint white line, mixed with some metallic blue scaling toward dorsum. Tornus with prominent black scalings. Fringe with white inner cilia, dark brown outer cilia. Abdomen: dark brown dorsad, white ventrad. Male genitalia ( Figures 14–16 View Figures 14–19 ): ring-shaped sclerites of 9+10 segments with width approximately 0.56 height, posterior end forming triangular flap dorsad of brachium; uncus a medial bump; saccus thin, flap-shaped; brachium simple, hook-shaped; valva broad, with prominent ridge ventrally; harpe forming a slightly concave cone-shaped bump; ampulla strongly curved, arm-shaped with posterior surface setose, distal end bifurcate, forming a thick, dorsal lobe with truncated end and a thin, tougue-shaped, ventral lobe. Phallus slender, upcurved posteriorly with pointed caudal end; aedeagus approximately 1.36 phallobase, cornuti absent. Juxta narrow, nearly circular but open dorsad.
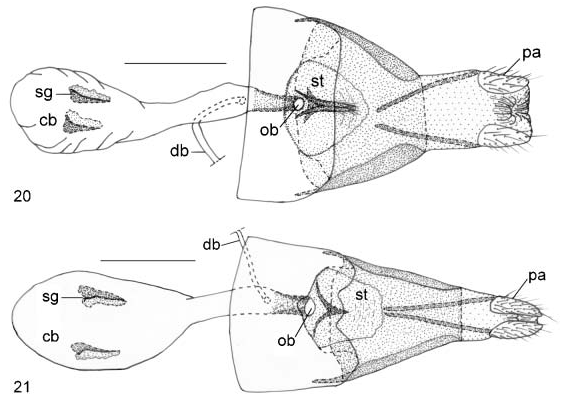
Female ( Figures 4, 5 View Figures 2–9 ): FL 16.4–17.6 mm (mean 17.2¡ 0.5 mm, n 54); AL 6.8–7.6 mm (mean 7.3¡ 0.3 mm, n 54). Body, wing patterns as described for male except scalings of underside buff, forewing upperside with a couple of barely visible orange spots in proximal end of M 2 and centre of M 3. ‘‘Tail’’-like projection of hind wing longer than that of male (length 4.7–5.2, with mean 4.9¡ 0.2 mm, n 54). Anterior margin of eighth tergum forming elongate, digitate process. Genitalia ( Figure 20 View Figures 20, 21 ): papillae anales weakly sclerotized, oval, setose. Apophyses posteriores slender, elongate, slightly flattened at base. Sterigma forming a broad, dome-shaped, sclerotized plate containing a prominent medial, inverted, pin-like, heavily sclerotized process lying caudad and dorsad immediate of ostium. Terminal end of this process setose, bifurcate. Ductus bursae sclerotized caudad, with ductus seminalis joining immediately ahead of this sclerotized part. Corpus bursae oblong, bearing a pair of prominent, invaginated, axe-shaped signa
Immature stages: ovum ( Figure 10 View Figures 10–13 ) approximately 0.77¡ 0.02 mm in diameter, 0.49¡ 0.02 mm in height (n 515), spherical but slightly compressed, chorion densely covered with minute spicules, micropyle as a prominent depression dorsad, white in colour. Larva: first instar ( Figure 11 View Figures 10–13 ): body grey in colour with long, transparent primary setae, turning pale green by first moult. Legs brown. Head, T1 shield, anal lobe dark brown. T1 shield ladder shape, anal lobe pentagonal. Primary setae as follows: on T1 shield, XD 1 longer than XD2, D1 longer than D2, SD1 and SD2 not visible. L1, L2, L3 forming a straight horizontal line. On T2 and T3 , D1 longer than D2, SD1 minute, SD2 absent, D1, D2 and SD1 forming a straight vertical line. L setal group forming a straight tilt line. On A 1–A6, D1 longer than D2, SD1 and SD2 minute, both above spiracles. L2 shortest in L setal group, anterodorsad of L1 and L3, L1 longest. On A 7 and A8, D2, SD1, SD2 absent. L setae as those of A1–A6. SV setal group bisetose on T1 , unisetose on T2 –A8. Second to fourth (final) instar ( Figure 12 View Figures 10–13 ): head brown with dorsal half pale brown tinged with yellow. Cranial sutures white. Body densely covered with short, brown or transparent secondary setae. Ground colour green with lateral, yellow shevrons, prominent yellow, longitudinal double lines present dorsad toward maturity. T1 shield, anal lobe turning green. Spiracles white. Newcomer’s organ and eversible tentacles absent. Body colour turning dark green tinged with blue upon pupation. Full-grown larva 15.9¡ 0.65 mm in body length (n 56). Pupa ( Figure 13 View Figures 10–13 ): of typical lycaenid type, surface wrinkly, covered with short brown setae. Ground colour pale brown mottled with dark brown markings. A pair of prominent dark brown dots present on T2 dorsad. A longitudinal medial band present on abdomen dorsad. Pupal length approximately 10.32 SD 0.34 mm (n 56) .
Phenology. Adults have yet to be observed in the wild. Field-collected larvae emerged in June.
Bionomics. The egg is laid near the base of the dormant buds of the host, approximately 30 cm up to 4 m above the ground. Larvae eclose in spring, devouring only soft tissues of the host. Older larvae possess the midrib-cutting behaviour shared by many Theclini lycaenids ( Koiwaya 1996a; Hsu 2002; Hsu and Liu 2002). Larva pupated under fallen leaves under laboratory conditions.
Diagnosis. Based upon genitalic structure, Teratozephyrus nuwai Koiwaya (1996b) ( Figures 6–9 View Figures 2–9 ) from western China is evidently the sister species of T. elatus ; both species share a shallowly bifid ampulla at distal end of valva and single, short, medial bump-like uncus in male ( Figures 14–19 View Figures 14–19 ). Teratozephyrus arisanus (Wileman, 1909) and T. tsukiyamahiroshii ( Fujioka, 1994) , plus all species of the closely related Esakiozephyrus and Iwaseozeohyrus Fujioka, 1994 possess a bifurcate uncus. The unci of the remaining described Teratozephyrus species are elongate ( Fujioka 1994), in contrast to the short, bump-like condition found in T. nuwai and T. elatus . Teratozephyrus elatus can be distinguished from T. nuwai by: (1) there are a pair of prominent orange spots on the fore wing upperside in both sexes of T. nuwai ( Figures 6, 8 View Figures 2–9 ), whereas those spots are greatly reduced or obsolete in T. elatus ( Figures 2, 4 View Figures 2–9 ); (2) ground colour of wing underside is buff in both sexes of T. nuwai ( Figures 7, 9 View Figures 2–9 ), whereas it is sexually dimorphic in T. elatus , buff in female ( Figure 5 View Figures 2–9 ) but grey in male ( Figure 3 View Figures 2–9 ); (3) inverted, funnel-shaped process of sterigma is longer than that of signum, with its caudal end setose and bifurcate in T. elatus ( Figure 20 View Figures 20, 21 ), whereas it is shorter than that of signum, with caudal end pointed in T. nuwai ( Figure 21 View Figures 20, 21 ); and (4) signa are axe-shaped in T. elatus ( Figure 20 View Figures 20, 21 ), in contrast to sickle-shaped in T. nuwai ( Figure 21 View Figures 20, 21 ). Within Taiwan, only Teratozephyrus yugaii is superficially similar to T. elatus in appearance, but their genitalic structures indicate they are not the most closely related to each other. They can be distinguished by the following characters: (1) the edges of the white lines on the wing undersides are uneven in T. elatus , but even in T. yugaii ; (2) outer cilia of hind wing is white in T. elatus , whereas it is buff in T. yugaii ; (3) distal end of valva is shallowly bifid in T. elatus , but deeply bifurcate in T. yugaii ; (4) uncus of T. elatus is short and bump-like, whereas it is prominently protruded and digitate in shape in T. yugaii ; and (5) sterigma forms a domeshaped, sclerotized plate with a slender, pin-like medial process in T. elatus , whereas it forms an elongate, spade-shaped plate with a robust, triangular medial protrusion in T. yugaii .
Type material. Holotype: L, Taiwan: Hualian Co., Xiulin, Guanyuan , 2335 m, 16 May 2002, coll. Y. F. Hsu, emerged 3 June 2002, reared from Quercus spinosa (Hsu no. 02E 37, BMNH) . Paratypes: 2 L, same locality as holotype, 19 May 2000 , coll. Y. F. Hsu, C. C. Lu, C. Y. Hung, emerged 17/ 18 June 2000, reared from Quercus spinosa ( Hsu no. 00 E22, 1 L genitalia preparation YFH 1219 , NTNU); 1 R , 26 May 2001, coll. Y. F. Hsu, emerged 26 June 2001, reared from Quercus spinosa ( Hsu no. 01E46, NTNU); 1 L, 10 June 2001 , coll. Y. F. Hsu, emerged 27 June 2001, reared from Quercus spinosa ( Hsu no. 01F16); 1 L, 16 May 2002 , coll. Y. F. Hsu, emerged 3 May 2002, 2 R , emerged 5 May 2002, 1 R , emerged 6 May 2002, reared from Quercus spinosa (Hsu no. 02E37, BMNH, NMNS, IOZ) .
Additional material. 1 L, 1 R (wings distorted) , same locality as holotype, 19 May 2001, coll. Y. F. Hsu, C. C. Lu and C. Y. Hung, emerged 18 June 2000 (Hsu no. 00E22, genitalia preparation YFH 1234 L /1235 R) .
Other phytophagous insects associated with the sclerophyllous oak community
During our survey, we also found the following phytophagous species associated with sclerophyllous oak communities: Phyllonorycter sp. ( Gracillaridae ) (HSU 02E38) and a leaf beetle ( Chrysomelidae ) (HSU 02E39) on Q. spinosa , plus leaf mines of a species of Phyllonorycter (Gracillaridae) (HSU 02E40) and a Stigmella species ( Nepticulidae ) (HSU 02E41) on Q. ‘‘ tatakaensis ’’.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.