Dicaeum dayakorum, Saucier & Milensky & Caraballo-Ortiz & Ragai & Dahlan & Edwards, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4686.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:2C416BE7-D759-4DDE-9A00-18F1E679F9AA |
|
persistent identifier |
https://treatment.plazi.org/id/5F5CF534-8023-45B0-809F-0A6A5949A8D4 |
|
taxon LSID |
lsid:zoobank.org:act:5F5CF534-8023-45B0-809F-0A6A5949A8D4 |
|
treatment provided by |
Plazi |
|
scientific name |
Dicaeum dayakorum |
| status |
|
Dicaeum dayakorum , species novum Spectacled Flowerpecker urn:lsid:zoobank.org:act:5F5CF534-8023-45B0-809F-0A6A5949A8D4
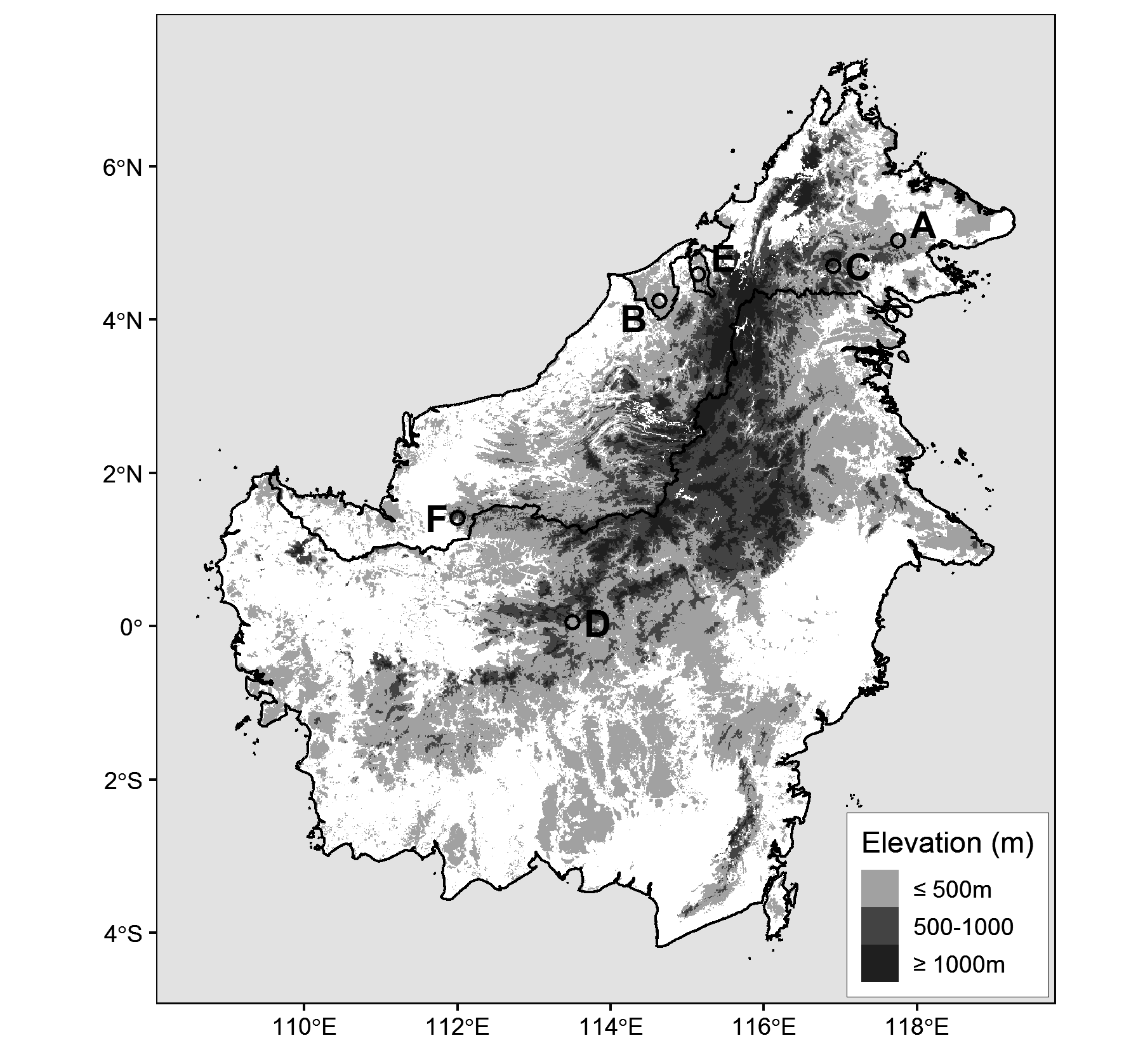
Holotype. —Study skin and partial skeleton, Smithsonian Institution, National Museum of Natural History , USNM 663246; tissues preserved in dimethyl sulfoxide (DMSO) buffer and later housed in gaseous nitrogen at the NMNH Biorepository (AK7DC13, AK7DC14, AK7DC15, AK7DC18); tongue, stomach and fecal samples saved; prepared by C.M.M., original field number CMM 5719; adult female; Malaysia: Sarawak; Lanjak Entimau Wildlife Sanctuary, 01°24’48”N, 112°00’16”, 350 m asl; GenBank sequences: MN416066 View Materials (ND2), MN416068 View Materials (ND3), MN416067 View Materials (TGFb2).
Diagnosis. —Phenotypically assignable to the genus Dicaeum Cuvier, 1816 , by short thin bill, specialized (bifid and semi-tubular) tongue morphology ( Fig. 4 View FIGURE 4 ), and a greatly reduced outermost primary feather ( Mayr & Amadon 1947, Salomonsen 1960a,b, Morioka 1992). Diagnosable as distinct from other species of Dicaeum by the following combination of characters; (1) rows of white orbital feathers above and below the eye, forming thin, but conspicuous white arcs; (2) entirely gray and white plumage coloration with no evidence of carotenoid pigments in the adult plumage; (3) short distal bill length (6 mm from nares to bill tip). Field observations indicate that the eye-arcs of putative males are even more strongly expressed than in the female holotype. The strong white eye-arcs of D. dayakorum are unique in Dicaeidae , although traces of pale eye-arcs are known to be present in female and juvenile plumages of a few other species (e.g. D. monticolum , D. agile , D. pygmaeum ). Red and yellow carotenoid pigments are evident in the plumage of most species of Dicaeum . The apparent lack of these pigments in the adult female holotype and field observations of putative adult males sets D. dayakorum apart from most of its congeners.
Description of Holotype.— Alphanumeric codes follow Munsell (1990) and capitalized color names are approximate and follow Smithe (1975). Upperparts (forehead, crown, back, rump) Dark Neutral Gray (N3.5 /0) becoming less dark on cheeks (N4/0) and darker on wing coverts and wing remiges (N3/0). Eye arcs clean white and conspicuous, but thin, extending only marginally past the first row of orbital feathers. Supraloral line faded-white extending from just past the orbital to the base of the nares. Submoustacial stripe faded-white, becoming more diffuse away from the bill. Malar and sides of throat Medium Neutral Gray (N5/0). Center of throat white, forming a stripe that narrows slightly below the throat (to 3.5mm wide) and extending down to the vent. Sides and flanks Medium Neutral Gray (N5/0) with pale-brownish undertones. Pectoral tufts and wing lining clean white. Thighs Dark Neutral Gray (N3.5 /0) with pale edgings. The white coloration from the ventral line extends onto the undertail coverts, where it is indistinctly mottled or stained darker. Ventral surface of rectrices Dark Neutral Gray (N3/0), with dorsal surface darker, almost appearing black (N2.5 /0).
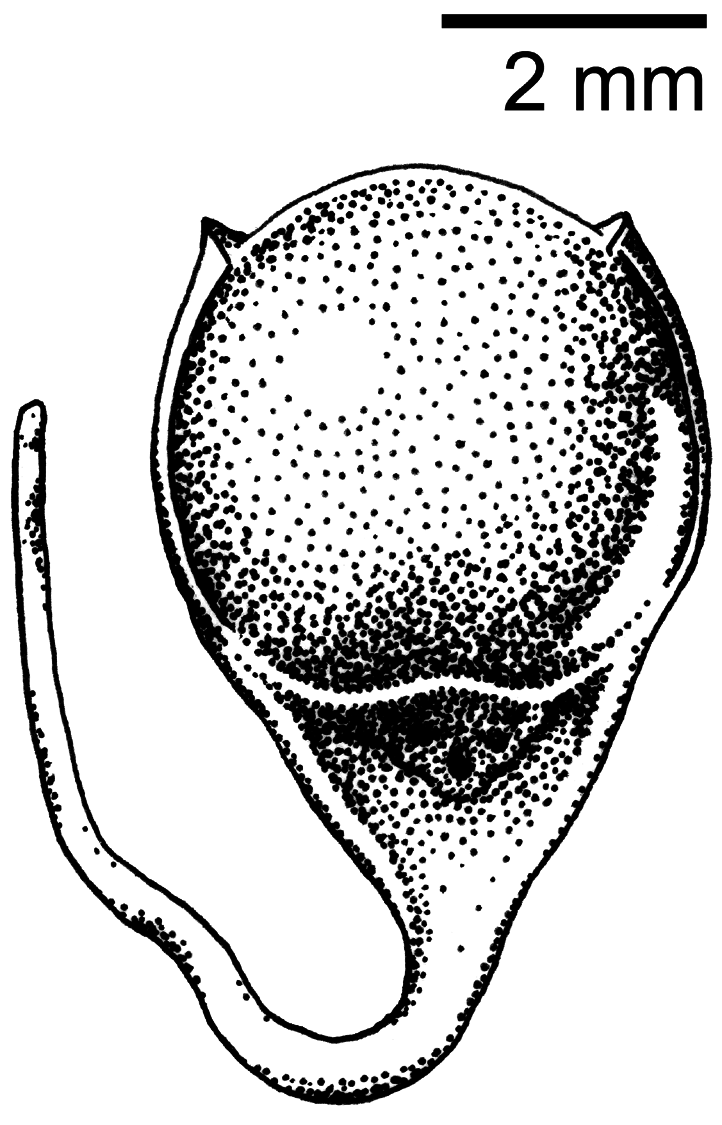
Soft part colors photographed and recorded at the time of collection: irides dark brown, maxilla black, mandible gray with dark tip, tarsi and feet black. No molt, little fat, skull 100% pneumatized, ovary 6 x 4 mm and granular, oviduct slightly enlarged, no bursa of Fabricius present. Stomach contained parts of a small jumping spider ( Salticidae ). Mistletoe seeds ( Loranthaceae ) were recovered from the lower large intestine ( Fig. 5 View FIGURE 5 ).
Measurements: mass 7.8 g; tarsus length 11.8 mm; wingchord 48.4 mm; tail length 24 mm; length of tenth primary 5.5 mm; bill width 3.6 mm; bill depth 3.3 mm; bill length from nares 6.0 mm; exposed culmen 8.0 mm (Appendix Table 1).
Paratypes.— No specimens other than the holotype were obtained and no additional specimens are known to exist.
Etymology.— We name Dicaeum dayakorum in honor of the Dayak people of Borneo. Their immense knowledge of the flora and fauna of their homeland forests is irreplaceable and crucial to future conservation efforts of Borneo’s endemic ecosystems.
The English name of ‘Spectacled Flowerpecker’ is assigned as proposed by Edwards et al. (2009), and refers to the broken eye-ring that is this species’ most characteristic and easily recognized plumage feature. This name also has the benefit of established usage in the ornithological and birdwatching community.
REMARKS
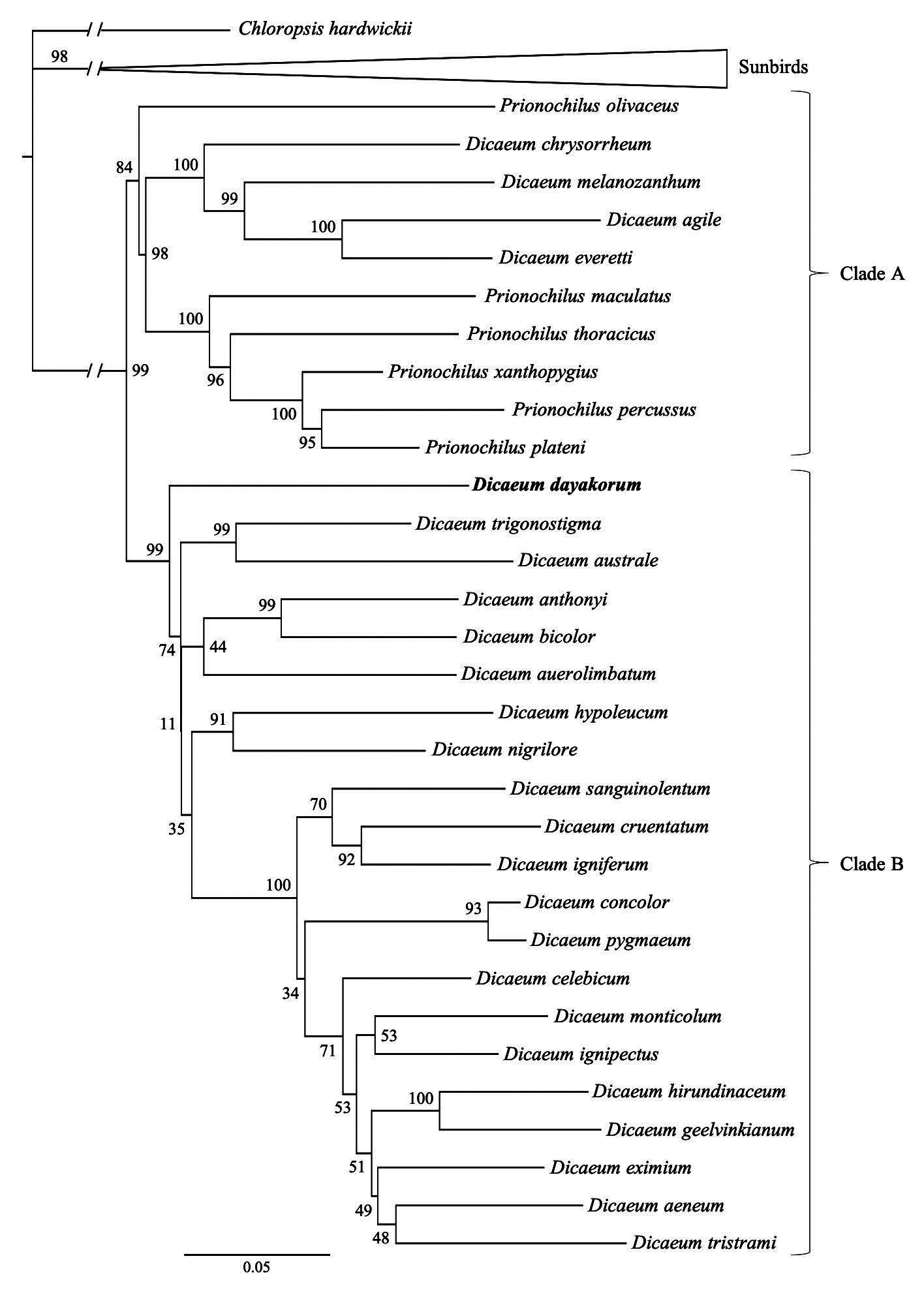
Phylogenetic relationships. —To infer phylogenetic placement of D. dayakorum within the Dicaeidae we sequenced DNA from the holotype to place it in the framework of a previous phylogenetic study of the family by Nyári et al. (2009). We downloaded sequence data from that study posted to GenBank, which included the nicotinamide adenine dinucleotide dehydrogenase subunit 2 (ND2, 1034 bp), ND subunit 3 (ND3, 351 bp), and the transforming growth factor beta 2 (TGFb2, 542 bp) for 30 species of flowerpecker and 11 outgroup species (10 sunbirds and 1 leafbird; Appendix Table 2). Genomic DNA was isolated from DMSO preserved tissues of the holotype specimen (USNM 663246). The DNA extraction was performed at the Smithsonian Laboratories of Analytical Biology (LAB) using a fully automated phenol-chloroform genomic extraction machine (Gene Prep, AutoGen, Holliston, MA). PCR products were amplified for all three coding regions (ND2, ND3, and TGFb2) using GoTaq® G2 Hot Start Master Mix (Promega Corporation) with additional BSA (New England BioLabs, 50 mM stock solution) and magnesium chloride (Bioline, Biolase DNA Polymerase, London, UK). The annealing temperature for ND2 was 57°C, and 59°C for ND3 and TGFb2. PCR products were cleaned and visualized on a 1% agarose gel. Cycle-sequencing was performed using ABI Prism® BigDyeTM Terminator (v3.1 chemistry, ThermoFisher Scientific, Waltham, MA) for both forward and reverse directions. The products were purified using SephadexTM (GE Healthcare Bio-Sciences Corp., Pittsburgh, PA) spin columns before submitting for sequencing on an ABI 3730 xl DNA analyzer at the LAB facilities. Raw sequences were visualized and quality-trimmed using Sequencher (version 5.4.6; Gene Codes Corporation, Ann Arbor, MI).
Sequences of the holotype were aligned with the rest of the dataset (42 taxa in total) using the MAFFT plugin (version 7.388; Katoh & Standley 2013) implemented in Geneious Prime 2019.1.3 (https://www.geneious.com, accessed on 28 June 2019). The multiple sequence alignments for each of the three loci were visually inspected and manually curated when necessary. As ND2 and ND3 represent portions of the same mitochondrial gene, both matrices were concatenated into a dataset of 1,383 bp. The individual datasets for both ND and TGFb2 genes were trimmed to remove characters not suited for phylogenetic inference using BMGE ( Criscuolo & Gribaldo 2010) with a sliding window size of three, a maximum entropy threshold and gap rate cut-off of 0.5, and a minimum block size of five. We estimated nucleotide substitution models for individual genes using ModelFinder ( Kalyaanamoorthy et al. 2017) implemented in IQ-TREE (version 1.6.11 Nguyen et al., 2014). We then concatenated the multiple sequence alignments for both genes, resulting in a matrix of 1,909 bp, and performed a Maximum Likelihood analysis with partitions using IQ-TREE, allowing the program to find the best-fit partitioning scheme possible to merge partitions ( Chernomor et al. 2016). The Maximum Likelihood analysis was replicated with 1000 ultrafast bootstrap iterations ( Hoang et al. 2017) by resampling partitions and then sites within resampled partitions, as recommended by Gadagkar et al. (2005). To detect incongruences in the topology of trees built from different organelles (i.e., nuclear vs. mitochondrial sequences), we performed ML analyses using only the concatenated ND2 and ND3 loci representing mitochondrial DNA, and the TGFb2 representing nuclear DNA. We estimated models of nucleotide substitutions for each of these loci and conducted a ML analysis using IQ-TREE with 1000 bootstrap replications as described above. Bayesian Inference (BI) analyses were conducted in MrBayes (version 3.2.6; Ronquist et al. 2012) using the CIPRES Science Gateway web server ( Miller et al. 2010). The BI was conducted using two separate four Markov Chain Monte Carlo (MCMC) analyses with 10,000,000 generations and sampling every 1000 generations. The first 25% of sample trees (“burn-in period”) were removed and a majority rule (> 50%) consensus tree was built. We estimated pairwise sequence divergences of raw mtDNA from the ND2-ND3 concatenated matrix using the Kimura 2-parameter distance model ( Kimura, 1980) with a Gamma distribution (Gamma parameter: 5) in MEGAX (version 10.1.0 Kumar et al. 2018). We also conducted a Poisson tree processes (PTP) species delimitation test with a subset of the taxa including all species of Dicaeum and Prionochilus with Chloropsis as outgroup, using a MCMC analysis with 100,000 generations with a burn-in period of 50% and a thinning of 100 ( Zhang et al. 2013).
Topologies of the ML and BI phylogenetic trees largely mirror those found in Nyári et al. (2009), and place D. dayakorum at a basal position of the “core” Dicaeum clade (Clade B sensu from Nyári et al. 2009) with high support (99% bootstrap support and 1.00 posterior probability in the in the ML and BI analyses, respectively; Figure 3 View FIGURE 3 ). The topology from the concatenated ML tree is largely congruent with the trees built using the two individual genes. However, as each of these individual datasets have fewer phylogenetically informative sites, the resultant trees show several unresolved nodes with low support values, especially at deep branches. The only notable discrepancy is the weak placement of D. dayakorum as sister to D. australe (74% bootstrap support) in the tree constructed from the TGFb2 gene.
Morphology and voice. —We took standard morphological measurements from the holotype, along with several of the most closely related species (as per our molecular analyses), and similar/co-occurring species of Dicaeum for comparison (Appendix Table 1).
Classic taxonomic treatments of the Dicaeidae focused on morphological characters such as bill shape, tongue morphology, and relative length of the outermost primary. D. dayakorum displays a relatively small and slender bill similar to that of D. monticolum and D. cruentatum , and distal bill length is at the low end of the range for the genus. The culmen is moderately arched downward and slight serrations are present along the edge of the distal half of both maxilla and mandible. The base is relatively wide compared to the tip and rictal bristles are reduced.
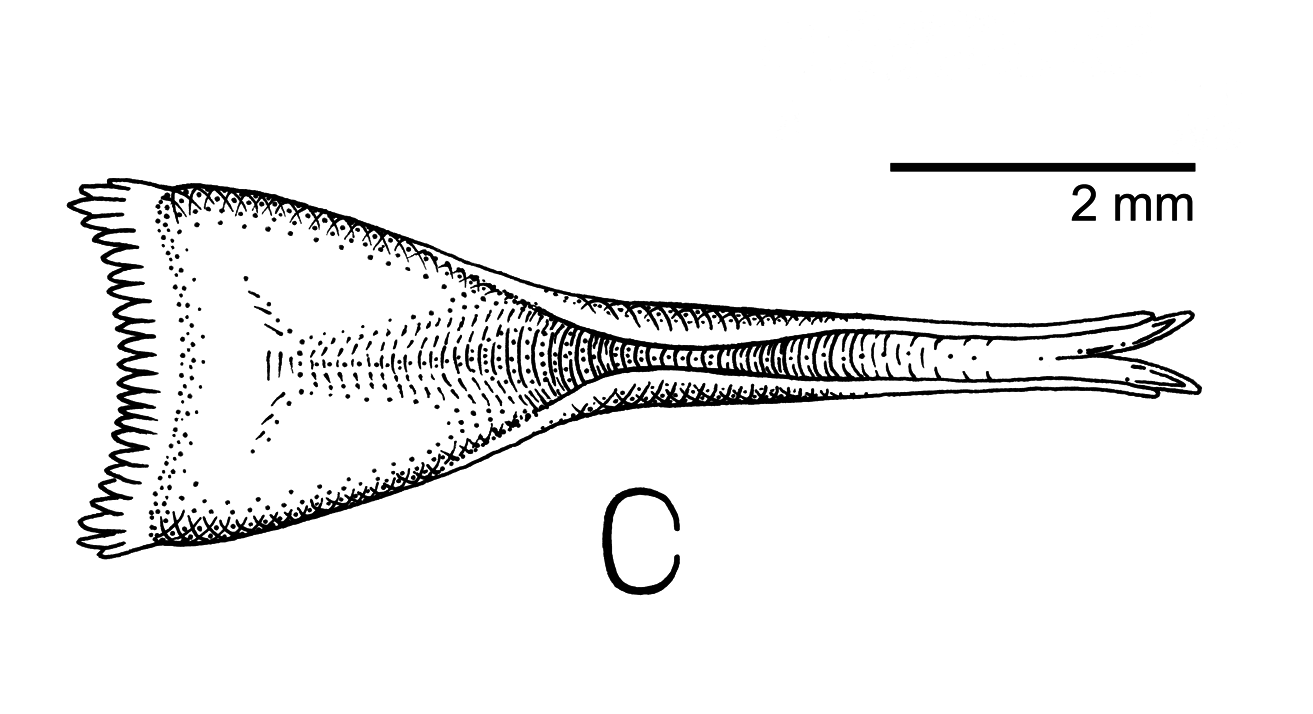
As opposed to the triangular, non-tubular tongues shown by members of Prionochilus, 1841 , many members of Dicaeum have specialized tongues with tubular structures and deeply bifid tips ( Morioka 1992). Tongue morphologies in this genus are often distinctive, reflecting varying degrees of specialization between frugivory and nectarivory ( Mayr & Amadon 1947, Morioka 1992). The tongue and hyoid of the holotype were preserved in aqueous formaldehyde and later transferred to ethanol. Examination revealed an overall structure that is not atypical for Dicaeum . The tongue has a triangular, fleshy base extending approximately halfway up the length of the structure giving way abruptly to a narrow, straight, and deeply concave anterior portion. This latter half is horny and semitubular at the base, becoming increasingly translucent distally, and ending in a strongly bifid tip ( Fig. 4 View FIGURE 4 ).
One of the most reliable characters differentiating flowerpecker genera has been the length of the 10 th or outermost primary, being relatively long in Prionochilus compared to Dicaeum ( Mayr & Amadon 1947, Salomonsen 1960a,b). Evaluation of this character in our holotype specimen revealed it to be greatly reduced (5.5 mm), fitting with the vestigial outer primaries possessed by other members of genus Dicaeum .
DPE heard the putative male sing with a series of ~12 high-pitched see notes, rising and then falling in pitch ( Edwards et al. 2009), but no recoding was obtained. Boyd et al. (2016) subsequently obtained the first and only known voice recordings of this species during their sightings of the birds in Central Kalimantan in 2015 (xeno-canto.org recordings XC239907, XC 301233). The recordings reveal a simple call typical of other flowerpecker species and an accelerating trilled song of 18 notes lasting 1.5 seconds, starting with two notes at 4.8 kHz, then immediately accelerating to a trill that rises from 5.3 kHz to a maximum centered at 6.1 kHz (5.8-6.4 kHz), and falls for the last three notes to 5.7 kHz. The song thus bears similarities, but is distinctive, to other known flowerpecker songs.
Taxonomy. —Clear phenotypic distinctiveness from known species of flowerpeckers, and high confidence values from phylogenetic and species delimitation analyses of our molecular dataset, strongly support Dicaeum dayakorum as a new species.
Nyári et al. (2009) recognized two predominant molecular clades in the Dicaeidae : generally thicker-billed species with a longer outermost primary (Clade A); and generally more slender-billed species with shorter outer primaries (Clade B). These clades are roughly concordant with the two established genera of flowerpeckers, Prionochilus and Dicaeum , respectively, with the exception of four traditionally Dicaeum species that tend to group with Clade A ( Nyári et al. 2009). Our independent analyses of the same molecular dataset (with the addition of USNM 663246) mirrors this arrangement, with strong support for D. dayakorum as sister to the core Dicaeum (Clade B; Fig. 3 View FIGURE 3 ). With the added consideration of morphological characters (e.g. outer primary length and tongue morphology) that are closely (though not exclusively) associated with the core Dicaeum lineage, we strongly support the view of Edwards et al. (2009) that the new species be placed in this genus.
We recovered no clear sister taxon to D. dayakorum from our phylogenetic analyses ( Fig. 3 View FIGURE 3 ). Although D. dayakorum seems to be sister to the rest of Clade B with high support (99% ML; 1.0 BI), branches separating this and related taxa are short and support values are low ( Fig. 3 View FIGURE 3 ), indicating that more molecular data may be needed to define this relationship satisfactorily. Results from the pairwise distance analysis suggest that genetic distances between D. dayakorum and other basal members of Clade B are high, ranging from 14–16% ( D. anthonyi : 16.1%; D. auerolimbatum : 16.8%; D. australe : 16.8%; D. bicolor : 15.6%; and D. trigonostigma : 14.5%). Species-level distinctiveness was further supported by the species delimitation test with a posterior probability support value of 0.99. In fact, the PTP analysis suggest that D. dayakorum is more distinctive at the molecular level than the rest of the 30 other flowerpecker taxa included in the Nyári et al. (2009) study. Although further taxon sampling is warranted, no unsampled species is a realistic candidate for conspecific status, and thus the proposed status of D. dayakorum as a new species will not be affected.
Ecology and distribution. —The type locality for Dicaeum dayakorum (USNM 663246) was characterized by tall, mixed dipterocarp forest upslope from the Nanga Segerak field station (LEWS, 350m asl). This section of forest lies at the edge of an expansive sanctuary dominated by primary lowland forest that has previously been subjected to light selective logging by local communities ( Chai 1996). The immediate area of capture was a relatively open and steep section of ridgeline, possibly the site of a previous treefall or landslide in the advanced stages of succession. The location seemed to be a crossing point for birds cresting the ridge and several bird species typical of canopy or upperstory flocks were observed here near ground level (e.g Irena puella, Psilopogon chrysopogon, Coracina fimbriata ). Documented observations depict a species with affinities for mature forest at lower elevations (30–350 m) ( Edwards et al. 2009, Sykes & Loseby 2015, Boyd et al. 2016). The elevation of the sightings in the Maliau Basin are unclear (likely between 260-1100 m), but could potentially represent higher elevation limits for the species ( Sykes & Loseby 2015). The Spectacled Flowerpecker has not yet been observed in areas with significant anthropogenic disturbance and we suspect it may be very rare or absent in heavily selectively logged forest and will abandon largely deforested areas.
Flowerpeckers are known to feed on a wide variety of fruits, flowers, and invertebrates ( Cheke et al. 2019). However, specialization on mistletoe fruit is one of the characteristic traits of the family, and is likely reciprocal due to their role as canopy seed dispersers ( Ali 1931, Kannan 1966, Cheke et al. 2019). Most, if not all, flowerpecker species possess gut adaptations thought to be specifically evolved for processing mistletoe, in which the rapidly digestible berries are able to bypass the muscular ventriculus so that the seeds can be expelled intact ( Desselberger 1931, Cheke et al. 2019). Many of the reported observations of D. dayakorum show it foraging in or closely associated with various species of mistletoe, often engaging in pulp-predation behavior ( Boyd et al. 2016). A review of available photographs from these observations revealed several mistletoe species from at least two families ( Loranthaceae : Macrosolen cochinchinensis ; Santalaceae : Ginalloa arnottiana Korth. and Viscum ovalifolium DC). Examination of the holotype’s gut contents found a partially digested jumping spider ( Salticidae sp.) in the gizzard as well as mistletoe seeds in the large intestine ( Fig. 5 View FIGURE 5 ). These seeds were identified as a species of Loranthaceae upon morphological examination. However, a BLAST search of its chloroplast DNA was inconclusive. Further investigation is needed to determine which of the over 16 genera of Loranthaceae reported for Borneo can be a suitable match.
The degree to which D. dayakorum is dependent on mistletoe is an important question that remains to be evaluated. The partially tubular tongue structure ( Fig. 3 View FIGURE 3 ), the presence of arthropod material in the stomach, and observations of the species foraging on Medinella (Melastomataceae) berries suggest a diet similar to that of other flowerpeckers in which a mistletoe-dominated diet is supplemented to some extent by nectarivory, general frugivory, and arthropod predation. The role of diet specialization on habitat preference and distribution is poorly known ( Reif et al. 2016). The various species of mistletoe that the bird has been observed to consume are ubiquitous species of Southeast Asia that likely occur widely throughout Borneo where mature forests persist ( Beaman et al. 2001, 2004). However, the patchy distributions of mature mistletoe plants ( Aukema 2004), and seasonality of fruit ( Barea & Watson 2007) suggest that the distribution of this resource is likely sparse and ephemeral. These factors, along with competition for fruit resources from other mistletoe specialists—especially other species of flowerpeckers as noted in the initial report by Edwards et al. (2009) —may have a limiting effect on population density. All other aspects of the bird’s life-history, including breeding biology, phenology, and vagility, remain undocumented.
Conservation. —Extrapolation of range based on documented localities of occurrence ( Fig. 1 View FIGURE 1 ) and the bird’s currently known foraging ecology suggest that D. dayakorum has a widespread distribution, with the potential to occur anywhere in the extensive lowlands of Borneo where suitable habitat exists. The pattern of occurrences suggests a bird that is patchily distributed, possibly even nomadic while following shifts in an ephemeral food source. It is also possible that the core distribution of the species has yet to be discovered. However, the considerable scarcity of these observations, despite documented occurrence in relatively well-explored areas (e.g. Danum Valley) is more difficult to explain and may be compounded by low population densities and difficulty of detection owing to the bird’s small size, dull coloration, and canopy dwelling tendencies.
Given our current lack of information on processes governing the distribution of this species, the potential effects of habitat fragmentation and disturbance should not be underestimated. However, our discovery of the species in Lanjak Entimau Wildlife Sanctuary—an approximately 187,000 ha. tract of protected lowland/hill forest managed by the Sarawak Forestry Corporation—is a reason to be hopeful ( Guntavid et al. 1997). We see no reason why D. dayakorum would not occur throughout the sanctuary’s pristine forests, or the contiguous Batang Ai National Park to the south. Although further surveys of the area are needed to assess habitat and mistletoe prevalence. Lowland forests such as this are the heart of Borneo’s ecosystems. Sadly, these sanctuaries are under increasing threat with the encroachment of intensive selective logging and unsustainable agricultural practices, including widespread conversion of lowland forest to oil palm ( Wilcove et al. 2013). It should also be noted that the indigenous Iban Dayak inhabitants of the region are the primary facilitators of conservation upkeep and protection. The imperilment and continued diminishment of these traditional longhouse communities is an underappreciated threat to the protection of these vital areas.
Many questions remain regarding the distribution, population dynamics, and ecology of the Spectacled Flowerpecker. Though the species may have once enjoyed a historical distribution of relative continuity throughout the lowland forests of Borneo, its current distribution has almost certainly become increasingly fragmented and diminished. We are hopeful that the formal scientific description of this exciting new species will help to underscore the importance of Borneo’s lowland forests as an area of ecological significance and future discovery.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.