Mormopterus norfolkensis ( Gray 1839 )
|
publication ID |
https://doi.org/10.5281/zenodo.184082 |
|
DOI |
https://doi.org/10.5281/zenodo.5613717 |
|
persistent identifier |
https://treatment.plazi.org/id/03928791-5A17-FF86-FDAE-E116EF9EFED3 |
|
treatment provided by |
Plazi |
|
scientific name |
Mormopterus norfolkensis ( Gray 1839 ) |
| status |
|
Mormopterus norfolkensis ( Gray 1839) View in CoL ( Figures 11–16 View FIGURE 11 View FIGURE 12 View FIGURE 13 View FIGURE 14 View FIGURE 15 View FIGURE 16 )
East coast free-tailed bat; Norfolk Island freetailed bat.
Note on the species name. Gray’s (1839) original spelling was norfolkensis but he used norfolcensis in Gray (1843) and both in Gray (1875). Both spellings appear in the literature until Tate (1952) after which the original spelling prevailed.
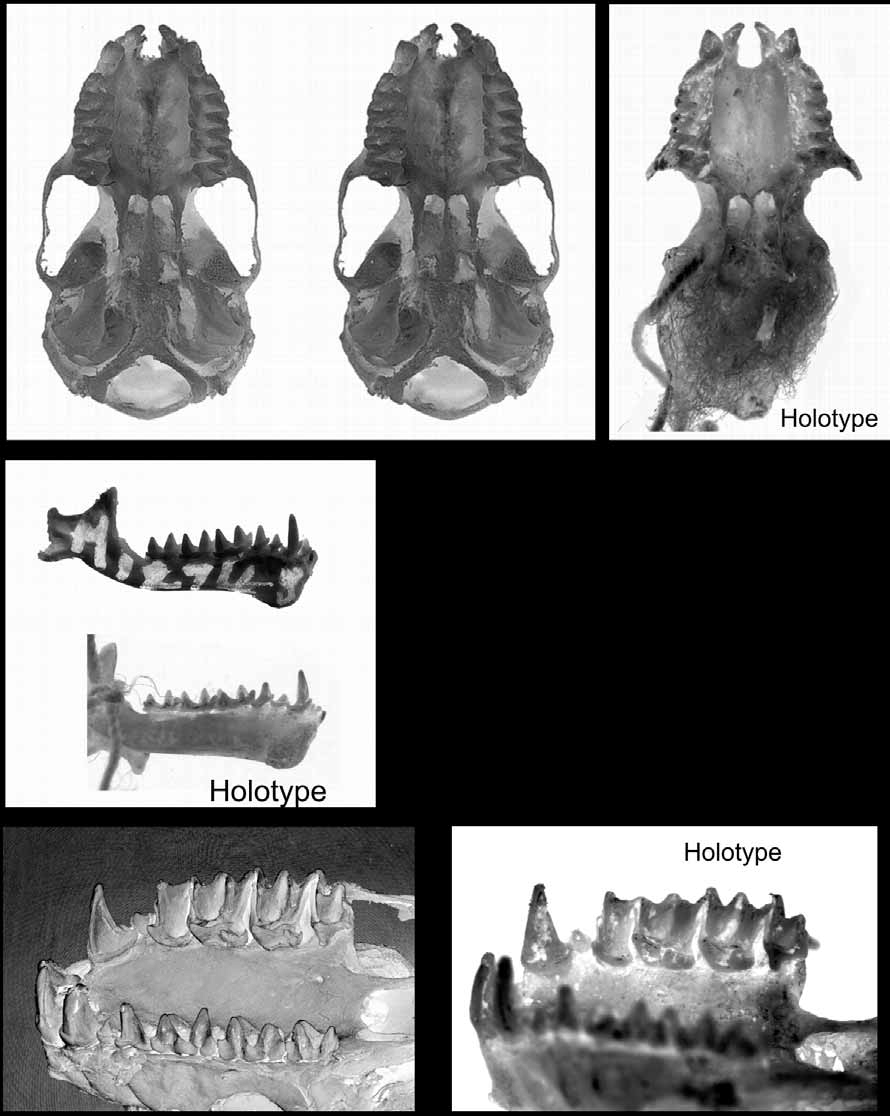
Holotype. Natural History Museum, London BM 1838.10.11.56. Sex not known (but probably female from this study). As dried skin, skull removed, rear of the skull damaged/missing.
Type locality. Possibly Norfolk Island, Australia (see above).
Referred specimens. New South Wales: AM M37640 View Materials d (Eden 36.941 0 S 149.906 0E); AM M12490φ (Kempsey 31.367 0 S 152.617 0E); AM M12754d AM M12748φ (Mumbulla State Forest 36.55 0 S 149.87 0E); AM M23663d (Kingswood, Sydney 33.75 0 S 150.717 0E); AM M30937 View Materials φ (near Myall Lakes National Park 32.5167 0 S 152.25 0E); AM M3280φ, AM M3281φ(Smithfield, Sydney 33.883 0 S 150.95 0E); AM M33584φ (Glossodia, Sydney 33.533 0 S 150.8 0E); AM M34486d (Cattai 33.55 0 S 150.917 0E); AM M5041d (Watson’s Bay, Sydney 33.85 0 S 151.283 0E); AM M37648 View Materials d (Badja State Forest 35.71 0 S 149.95 0E); ANWC M02321 View Materials ? (Orchid Hills, Sydney 33.8 0 S 150.75 0E); AM M37738d (Bodalla State Forest 36.11 0 S 150.08 0E); ANWC M00209 View Materials –15 4φ, 3d (Tooloom, Urbenville 28.5S0 152.433E0); AM M38976 View Materials d (Camden 34.04 0 S 150.680 E).
Queensland: QM JM9032φ, JM9037φ, JM9036d (Conondale Range 26.7 0 S 152.617 0E).
Diagnosis. Allozyme profile. M. norfolkensis differs at an average of 49 %FD from M. eleryi and from all other known Australian Mormopterus species by an average of 48 %FD at 40 putative allozyme loci ( Table 2b View TABLE 2 b ). M. norfolkensis is characterised by alleles not found in any other species at the following nine loci: Acon2, Ada, Ca, Dia, Got 2, Gpd, Guk, Ldh1 and Sod ( Table 2 View TABLE 2 b a).
Morphology. M. norfolkensis differs from all other examined Australian Mormopterus species except M. eleryi by having the proportion of M3-M3 width to forearm length less than 0.18 (0.167–0.174 cf 0.185–0.236) and proportion of C1–C1 width to proximal phalanx of digit3 length less than 0.28 (0.238–0.275 cf 0.288– 0.399).
M. norfolkensis differs from all other Australian Mormopterus species except M. eleryi by the possession of long slender fleshy genital projections in both sexes ( Figure 11 View FIGURE 11 ). The male projection arises from the distodorsal surface of the prepucial skin; the projection length ( 1.1–1.98mm) averages 60% of the length of the penis ( 2.08–2.91mm). In M. eleryi the length of the male projection length is 1.19–1.93mm while in the “ planiceps-beccarii-loriae complex” it is absent or never exceeds 0.8mm.
The female projection in M. norfolkensis arises from the anterior edge of the genital opening and is 1.76– 2.67mm in length M. eleryi it is 1.83–2.25mm. In the “ planiceps-beccarii-loriae complex” it is absent or less than 1.6mm in length.
M. norfolkensis is unique amongst Australian Mormopterus species by its possession of a glans penis with a distal claw ( Figure 11 View FIGURE 11 ).
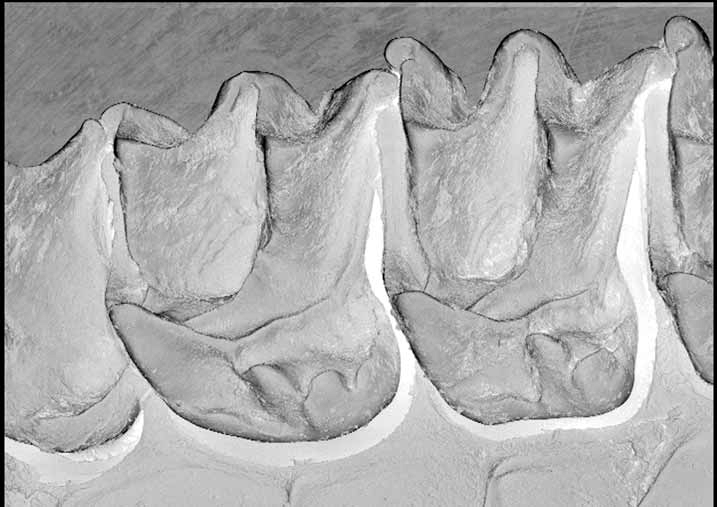
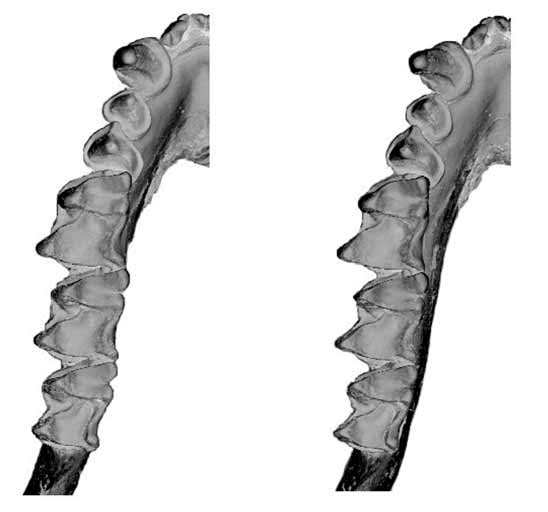
Differs from all other examined forms by the shape and structure of the upper first and second molars ( Figure 12 View FIGURE 12 ). In M. norfolkensis there is no typical hypocone, but rather a scallop-shaped extension of the posterolingual margin of the heel but allied to the same structure in M. eleryi . The upper molars of M. norfolkensis also differ markedly from M. eleryi as described above.
M. norfolkensis differs from Malagasy M. jugularis and Mascarene M. acetabulosus and Sumatran M. doriae by the absence of a gular sac and the possession of four rather than six lower incisors. Peterson (1985) claimed that the holotype of M. norfolkensis is sub-adult and should be regarded as synonymous with M. acetabulosus . We do not agree with either of these assertions. The norfolkensis holotype appears to be adult with wing epyphises that are fully fused and teeth that exhibit some wear. It differs from acetabulosus in regard to the incisors and the gular sac as mentioned above, as well as the structure of the upper molars and glans penis morphology.
M. norfolkensis differs from Cuban M. minutus , and South American M. phrudus and M. kalinowskii by the absence of a gular sac.
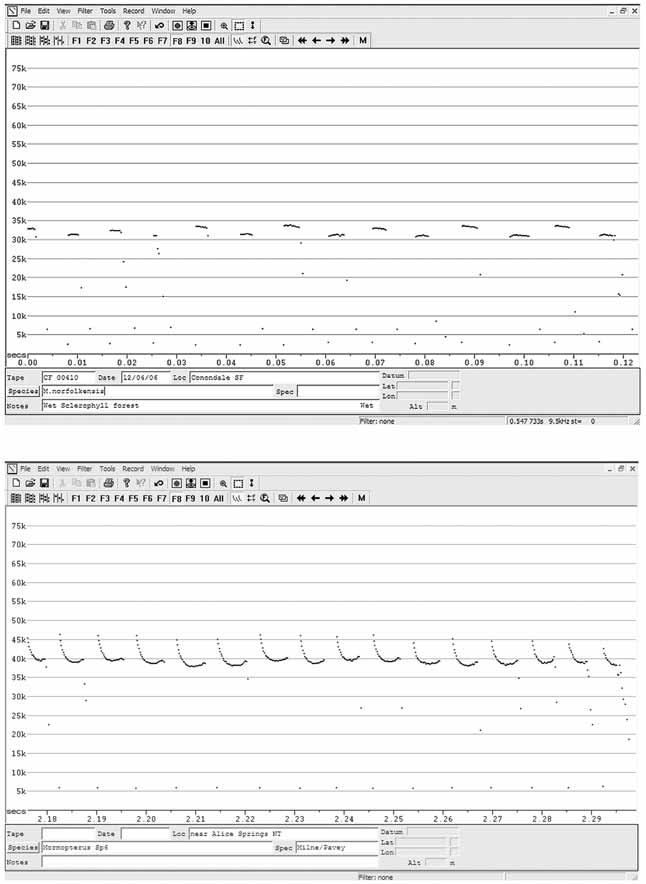
Echolocation call. The search phase echolocation call is very variable, but usually comprises short duration pulses of 2–4msec., usually of constant frequency but can be frequency modulated or slightly dome shaped and in the range of 30–35kHz. Many calls have alternating frequency pulses, the lower pulse anywhere between 1 and 4kHz displaced from the upper pulse ( Figure 3 View FIGURE 3 ). Even though the calls are variable, alternating frequency pulse calls are not known from any other Australian molossid species, thus making calls of M. norfolkensis readily identifiable.
Sympatry. M. norfolkensis is sympatric with Species 1, 2, 4 east, and within 150km of M. eleryi .
Description. Externals. A small but slight species weighing 7–10g and forearm length 35.7–38.6mm. The muzzle is small, tapered to the nostrils, thus differing from the “ planiceps-beccarii-loriae complex” where the muzzle is swollen. The nostrils are large and their margins stand proud of the muzzle. The outer margin of the rhinarium and the median line of the septum are coarsely crenulated. Face well covered with fine ginger-coloured hairs, especially forward of the eyes. There are short bristles on the side of the muzzle the length of which match the shortest in M. eleryi and are relatively uniform in length and less than 0.5mm. There are no long bristles on the top of the muzzle as in eleryi . The ears are triangular, rounded at the tips but acute; inner margins separate and divided by a small crop of fur. There is a distinct notch in the margin of the ear just preceding the very weakly developed antitragus. Tragus tip is rounded. There is no gular sac.
Dorsal fur with hair shafts up to 6mm in length, the distal 80% Cinnamon-Brown to Russet and the bases cream. Ventral fur lighter, shafts tricoloured with tips cream, central 80% Drab and bases cream.
Koopman (1984) has discussed the confusion in Hall and Richards (1979) in which they state in their key, that in norfolkensis “the wing attaches approximately 1/3 of the tibia’s length from the ankle” and then later in the text that “the wing arises from the ankle”. Troughton (1941) also used the ankle attachment of the wing as a diagnostic character for norfolkensis . We agree with Koopman’s view that the exact point of attachment is open to interpretation, and in our view, the point of attachment is not a particularly good character to define norfolkensis , especially when there are many unambiguous features that define the species.
The glans penis is very distinctive. Overall it is cigar-shaped, slightly dorso-ventrally compressed, and has a length of 2.26mm in the one individual measured (AM M12754) ( Figure 11 View FIGURE 11 ). Backward facing barbs cover almost the entire surface of the glans. The unique feature of the glans is the distal claw. The claw is broad at the base where it emerges from the glans just ventrally and slightly posterior to the glans tip. It curves upwards and tapers to a point just proud of the dorsal surface of the glans. The urethral opening is not clearly indicated and may be hidden behind the base of the claw. The function of this striking feature is not known. A reasonable speculation is that the claw is involved in the removal of vaginal plugs prior to copulation.
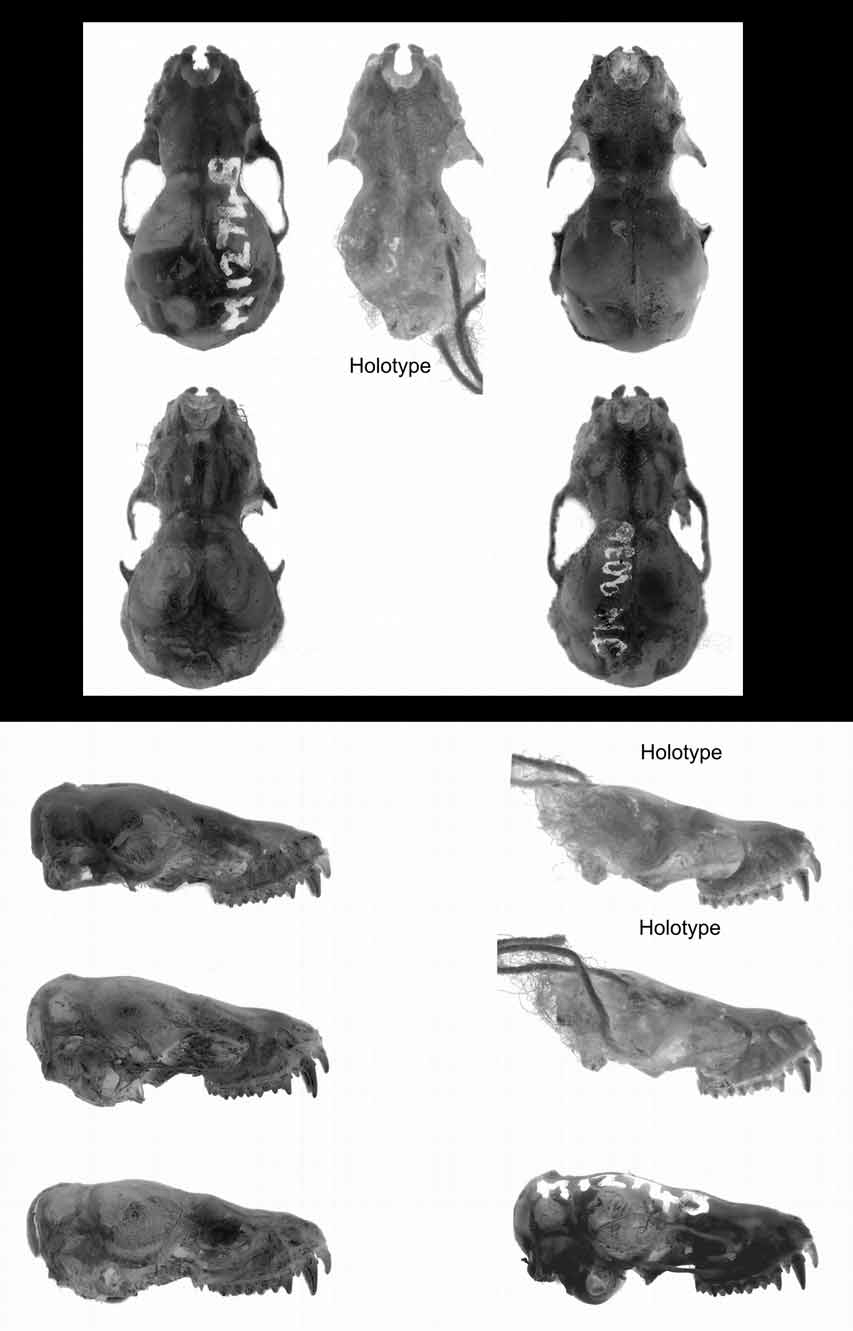
Skull and dentition. Comparison views of the holotype and mainland forms are shown in Figures 13–14 View FIGURE 13 View FIGURE 14 . The skull appears less robust than those of the “ planiceps-beccarii-loriae complex” and especially when compared to M. beccarii , a species of similar forearm length.
From the dorsal view and working from the rear, the supraoccipital is bulbous and inflated well outside of the line of the lambdoidal crests (missing in the holotype). The lambdoidal crests commence moderately but become insipid as they sweep forward to meet an equally poorly developed sagittal crest. But the junction (the lambda) is developed to form a small tetrahedral shaped protuberance. The parietals and interparietals are inflated leaving a diamond-shaped depression at their junction. This depression is overlaid with a rough bony deposit presumably to facilitate muscle attachment. The braincase is dorsally rounded. There is some variation in the development of the postorbital process. In AM M12748 the postorbital process is absent and the line of the supraorbital ridge is almost straight to the lachrymal process. In specimen JM 9032, there is small spicule on the sphenorbital projection and the supraorbital ridge is distinctly concave between this and the lachrymal process. The holotype also has an obvious postorbital process and a concave supraorbital ridge. The lachrymal process is well developed and when viewed dorsally, extends to the outer margin of the maxilla. The posterior margin of the narial emargination is large and rounded except for a small median projection.
From the lateral view, the profile is sinuate, with an elevated braincase and is unlike the flattened and straight profile in most of the " planiceps-beccarii-loriae complex".
From the ventral view, features include a high-domed palate, the glenoid not laterally developed to extend beyond the edge of the line of the braincase. The pterygoid wings are parallel, and the basisphenoid pits oblong, conspicuous but very shallow.
Dental formula: i1 /2 c 1/1 p2/ 2 m 3/3=30. Features of the upper teeth include a well-developed P2, the cingulum of which contacts the cingulum of the canine but has a small gap to the P4. In this respect the tooth row is less crowded than in other species. In both M1 and M2 the paraloph, metaloph and post protocrista are strongly developed and the protofossa very deep. The heel and hypocone structure differ considerably from that in both M. eleryi and the " planiceps-beccarii-loriae complex". The scanning electron micrograph ( Figure 12 View FIGURE 12 ) shows the structure in detail. On M1, the posterolingual margin of the heel is rounded from the base and curves occlusally to form a lip with 3–4 blade like lobes. The most mesial of these lobes is probably homologous with the typical hypocone of the " planiceps-beccarii-loriae complex" although its shape is not so conical. Between the posterolingual lip and the postprotocrista is an extensive basin. In M2 the heel is not so posteriorly developed and the posterolingual lip is less scalloped in appearance. It too has a hypocone and an extra lobe from the lip. The lingual-most lobe is similar in form to the crotchet hook-like structure in M. eleryi . The lower dentition is shown in Figure 15 View FIGURE 15 .
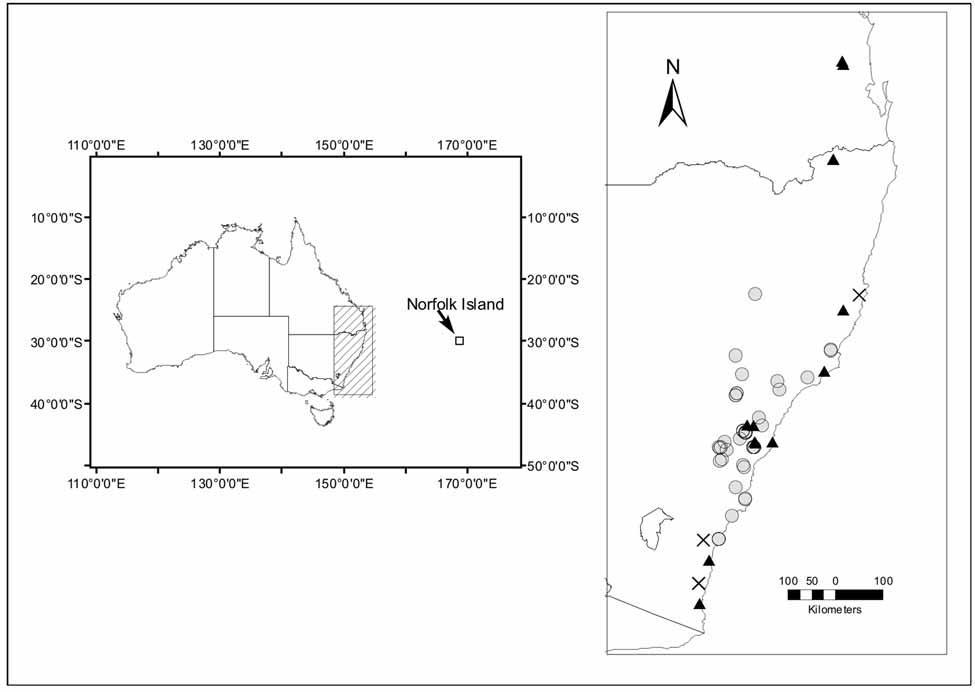
Distribution, biology and conservation status. The distribution map ( Figure 16 View FIGURE 16 ) presented here is based on the collection localities of the museum specimens used in this study, as well as records drawn from the New South Wales Department of Environment and Climate Change (DECC) fauna database. A map of the DECC norfolkensis records is accessible on the internet (http://wildlifeatlas.nationalparks.nsw.gov.au/wildlifeatlas/html/default.htm; NSW National Parks and Wildlife Service 2008). The DECC database contains mainly records from captured and released specimens and from echolocation recordings; many of these records are early records and many were generated from non-bat specialists. Such records are not verifiable and many are likely to be incorrect; therefore we have included only localities from those records submitted by experienced bat workers. The map ( Figure 16 View FIGURE 16 ) probably represents a good reflection of the extent of the distribution of M. norfolkensis but may underestimate the number of genuine records.
The mainland distribution is coastal, on the eastern side of the Great Dividing Range from Bega in southern New South Wales to the Conondale Range in southern Queensland. If M. norfolkensis did occur on Norfolk Island in the past, it is unlikely to occur there now ( Hoye 2007). Allison and Hoye (1995) and Churchill (1998) describe the habitat as ranging from dry eucalypt forest and woodland to wet sclerophyll and rainforest. Natural roosts are formed in hollow spouts in large eucalypt trees. Females give birth in late November or early December.
Although currently listed as Vulnerable in New South Wales (Threatened Species Conservation Act 1995 No 101), M. norfolkensis is not listed under any threatened category in Queensland or under federal legislation. This distribution coincides with the most densely human populated region of Australia.
| ANWC |
Australian National Wildlife Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |