Micrurus obscurus ( Jan, 1872 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4668.3.4 |
|
publication LSID |
lsid:zoobank.org:pub:D5705B5C-EB6B-4DB3-85A3-279898999DD1 |
|
persistent identifier |
https://treatment.plazi.org/id/0392C527-C74A-FF92-ADF3-FA3F202AB9B0 |
|
treatment provided by |
Plazi |
|
scientific name |
Micrurus obscurus ( Jan, 1872 ) |
| status |
|
Micrurus obscurus ( Jan, 1872) View in CoL
Elaps corallinus var. obscura Jan in Jan & Sordelli 1872:5 .
Elaps heterozonus Peters 1881 , Sitzber. Ges. naturf. Freunde, Berlin. Sarayacu, Ecuador: 52.
Elaps princeps Boulenger 1905 , Ann. Mag. Nat. Hist. Sara, Sta. Cruz de la Sierra, Bolivia: 456.
Micrurus spixii obscura Schmidt & Walker 1943:294 .
Micrurus spixii obscurus— Schmidt 1953:175.
Micrurus spixii princeps Schmidt 1953:175 .
Micrurus obscurus — Harvey et al. 2003:22 .
Micrurus spixii obscurus — Campbell & Lamar 2004:229 .
Micrurus spixii princeps— Campbell & Lamar 2004:230.
Micrurus obscurus —Wallach et al. 2014:451 .
Micrurus princeps —Wallach et al. 2014:451 .
Micrurus spixii obscurus— Silva Jr. et al. 2016b:134.
Micrurus obscurus — Valencia et al. 2016:321 .
Holotype. Jan (1872) place “ Lima ”, supposedly in the Pacific coast of Peru as, the type-locality. Schmidt & Walker (1943) minted out that this origin was in error and suggests to restricting the type-locality to “East of Peru ” after analyzing specimens from the University of Arequipa. Schmidt (1953 b) restricted the type-locality to Iquitos, department of Loreto, Peru, claiming that the abundant presence of specimens in this region of the Amazon has characterized it as the main habitat for the species. The holotype ( MSNM 1872:41) illustrated by Jan & Sordelli (1872) was not found in the collection ( Fig. 2C View FIGURE 2 ). According to Harvey et al. (2003), the holotype may have been destroyed during World War II. When checking the reference collection, the specimen could not be found by the curator in charge (Stefano Scali), which reinforces the idea that it may have been lost. For this reason, we used as reference to the holotype only the figure contained by Jan & Sordelli (1872).
Diagnosis. Micrurus obscurus can be distinguished from all congeners by unique combination of the following characters: white snout with black-bordered scales; interocular black band separating white snout from red parietal region; gular region red with black-spotted scales on the posterior border; body triad 3–10; black ring of the first triad incomplete (MIBL), dorsally elongated (4–16 scales long) with anterior portion triangular; ventrals 197–228 in males, 202–226 in females; subcaudals 14–26 in males, 14–23 in females; last body triad incomplete; ANTWH of first triad narrow (2–4 scales long); hemipenis bilobed, partially capitate and with calcified spines arranged in diagonal rows, inconspicuous capitular crotch not dividing the organ completely and weakly developed basal pocket; premaxilla located below the nasal; posterior extremity of the parietal bone wide and rounded but does not exceed the height of the contact between the prooptic, and shorter fang with respect to maxillary.
Comparisons. Micrurus obscurus differs from M. spixii by having tricolor head with dark interorbital bar, white anterior ring before black ring of an incomplete triad, hemipenis non-capitate, and fangs proportionally short with respect to maxillary (vs. well-defined black cephalic cap connected to first black ring, hemipenis capitate, and venom fangs proportionally long with respect to maxillary); differs from M. diana by having absence of cephalic cap, first body triad incomplete, body triads 3–10, last triad incomplete, ventral 174–228 (vs. cephalic cap connected to first black body ring, first body triad complete, body triads 9–15, ventrals 214–116); differs from M. filiformis by having white snout with black-edged scales, relatively robust body, ventrals 197–228 and less than 11 complete body triads (vs. black snout interrupted by white transverse band, body thin, ventrals 239–329, and 10–22 complete body triads); differs from complex M. lemniscatus complex by having white snout with black-edged scales, infralabias without characteristic spots and ventrals 197–228 (vs. black snout, followed by white and black band, and inverted U-shaped spot covering infralabials; ventrals 225–263 in M. lemniscatus helleri ; ventrals 225–277 in M. l. lemniscatus ); differs from M. hemprichii ortoni by having divided cloacal scale and red rings composing triads (vs. entire cloacal scale and yellow rings composing triads); differs from M. nattereri and M. surinamensis by having white snout with blackbordered scales, anterior ring narrow and white before first black ring, first body triad incomplete, eyes and nostrils positioned laterally, and hemipenis short without papillate or spinulate calyces (vs. red snout with black posterior border, narrow anterior and posterior black ring as well as white rings, first body triad complete, eyes and nostrils oriented toward the top of head, and hemipenis short, moderately elongate with calyculated lobes and calcified spines).
Meristic and morphometric variation (n= 150). Ventrals 197–228 in males (mean= 214; SD= 6.7; n = 71), 202–226 in females (mean = 214; SD= 4.9; n = 58); subcaudals 14–26 in males (mean= 19.7; SD= 3.1; n = 67), 14–23 in females (mean= 18.9; SD= 2.2; n = 51); head length 8.1–43.3 mm in males (mean = 22.9; SD= 7.6; n = 73), 10.1–32.4 mm in females (mean= 20.2; SD= 6.6; n = 47); SVL 220–1260 mm in males (mean= 748.1; SD= 303.9), 220–1122 mm in females (mean= 640.8; SD= 262.6); TL 12.1–66.7 mm (mean= 40.1; SD= 14.6; n = 67) in males, 12.1–54.1 mm in females (mean= 33.8; SD= 13.7; n = 51); number of body triads 4.3–9.6 in males (converted: 2/3+3+1/3–2/3+8+2/3; mean= 6; SD= 1.12; n = 79), 4.3–11 in females (converted: 2/3+3+1/3–2/3+10+2/3, mean= 7; SD= 1.3; n = 57); ANTRD 6.8–53.9 in males (mean= 26.1; SD= 13.2; n = 70), 6.2–57.6 in females (mean= 25.5; SD= 14.1; n = 49); ANTBL 3.2–39.3 in males (mean= 16.8; SD= 8.2; n = 70), 4.1–29.9 in females (mean= 15.2; SD= 7.1; n = 49); ANTWH 4.9–53.1 (mean= 22.3; SD= 10.6; n = 70), 4.8–41.2 in females (mean= 19.8; SD= 9.5; n = 49); MIBL 3.9–53.5 in males (mean= 19.6; SD= 10.4; n = 70), 4.2–33.1 in females (mean= 16.4; SD= 7.5; n = 49); POSWH 3.5–46.2 in males (mean= 21.2; SD= 9.9; n = 70), 5.1–38.8 in females (mean= 19.2; SD= 9.2; n = 49); POSBL 3.9–40.6 in males (mean= 16.5; SD= 8.1; n = 70), 4.4–28.8 in females (mean=14.7; SD= 6.7; n = 49); POSRD 6.1–64.8 in males (mean= 25.3; SD= 13.5; n = 70), 4.2–54.2 in females (mean= 23.8; SD= 13.1; n = 49).
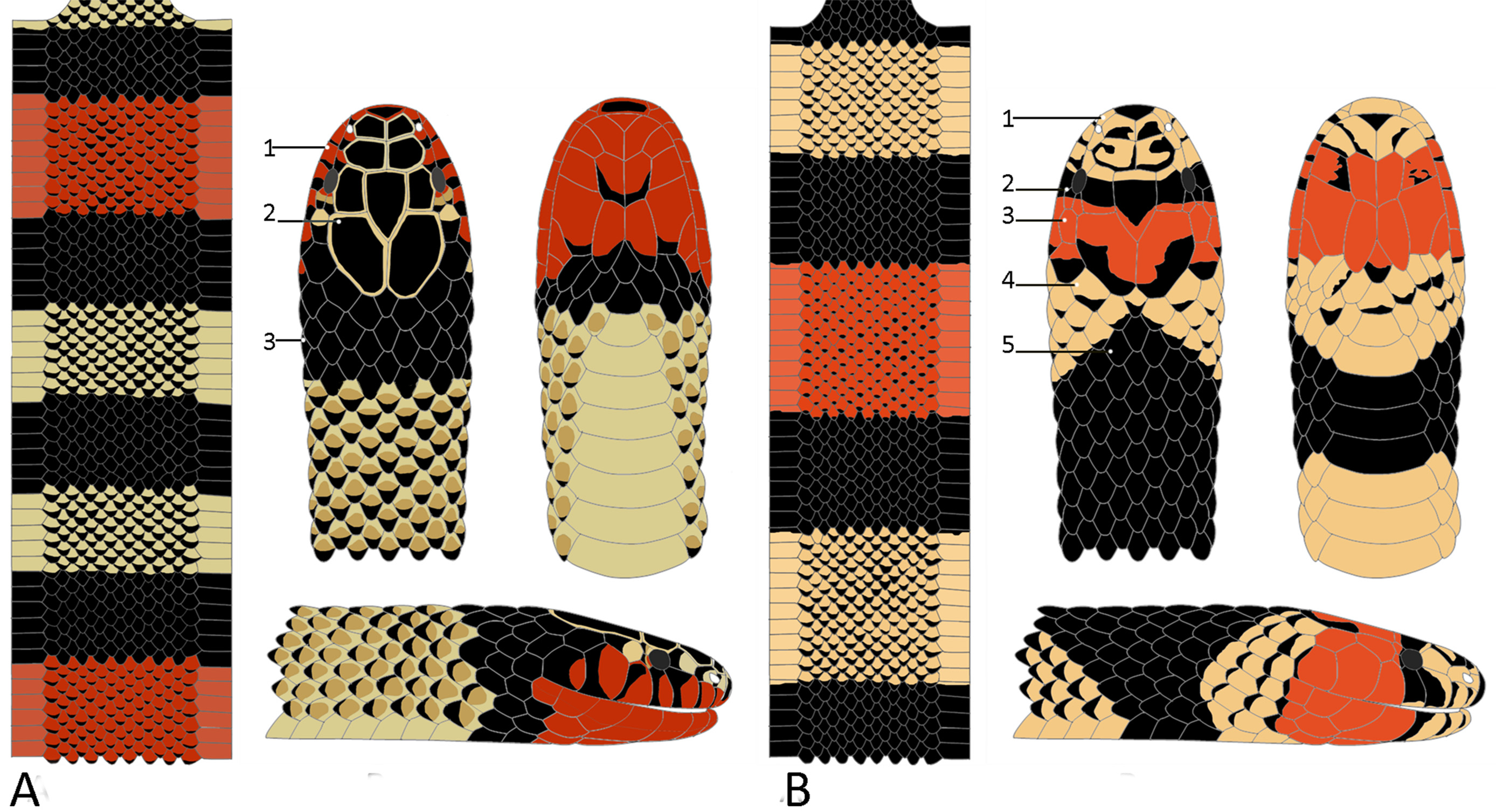
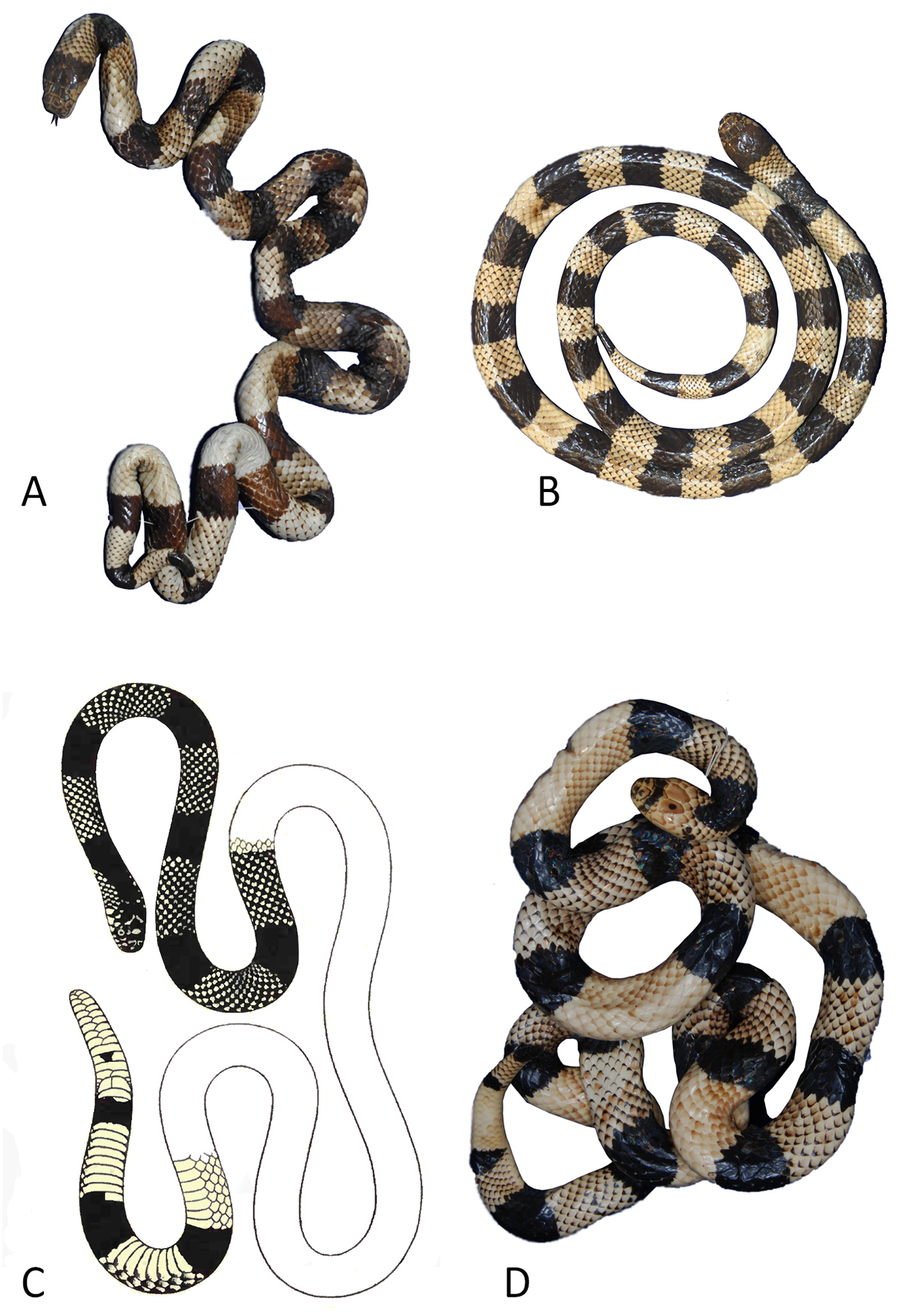
Color pattern ( Figs. 1B View FIGURE 1 , 10 View FIGURE 10 A-F). In life (n = 14) white snout formed by rostral, internasals, nasals, prefrontals, preoculars, supraoculars and frontal; cephalic scales with black posterior borders; dark interorbital bar with irregular contours, separating white snout from red cephalic region; red region starting at posterior border of frontal, covering parietals, postorbital, anterior temporals, and fifth to seventh supralabial; mental black, first and second infralabials red with black spots, third to fifth supralabial red with black borders; first triad of body incomplete followed by 3–10 complete triads; white rings with anterior borders darkening gradually to black posterior borders; black rings become narrower ventrally; red rings with brown posterior borders; first incomplete body triad with narrow ANTWH, followed by MIBL, POSWH and POSBL rings (2/3); tail triad incomplete (1/3) or complete; dorsal scales of white and red rings with black posterior borders, 2/3 of scales dark and gradually become lighter towards anterior border.
In preservative solution (n = 165) ( Fig. 10 View FIGURE 10 C–E), red becomes light buff and creamish white becomes pale buff. In melanic specimens (n= 19) ( Fig. 10B, D, F View FIGURE 10 ), darkening occurs in keratinized portion of cephalic and corporeal scales; specimens showed a strong tendency towards melanism 43.5% (n = 71).
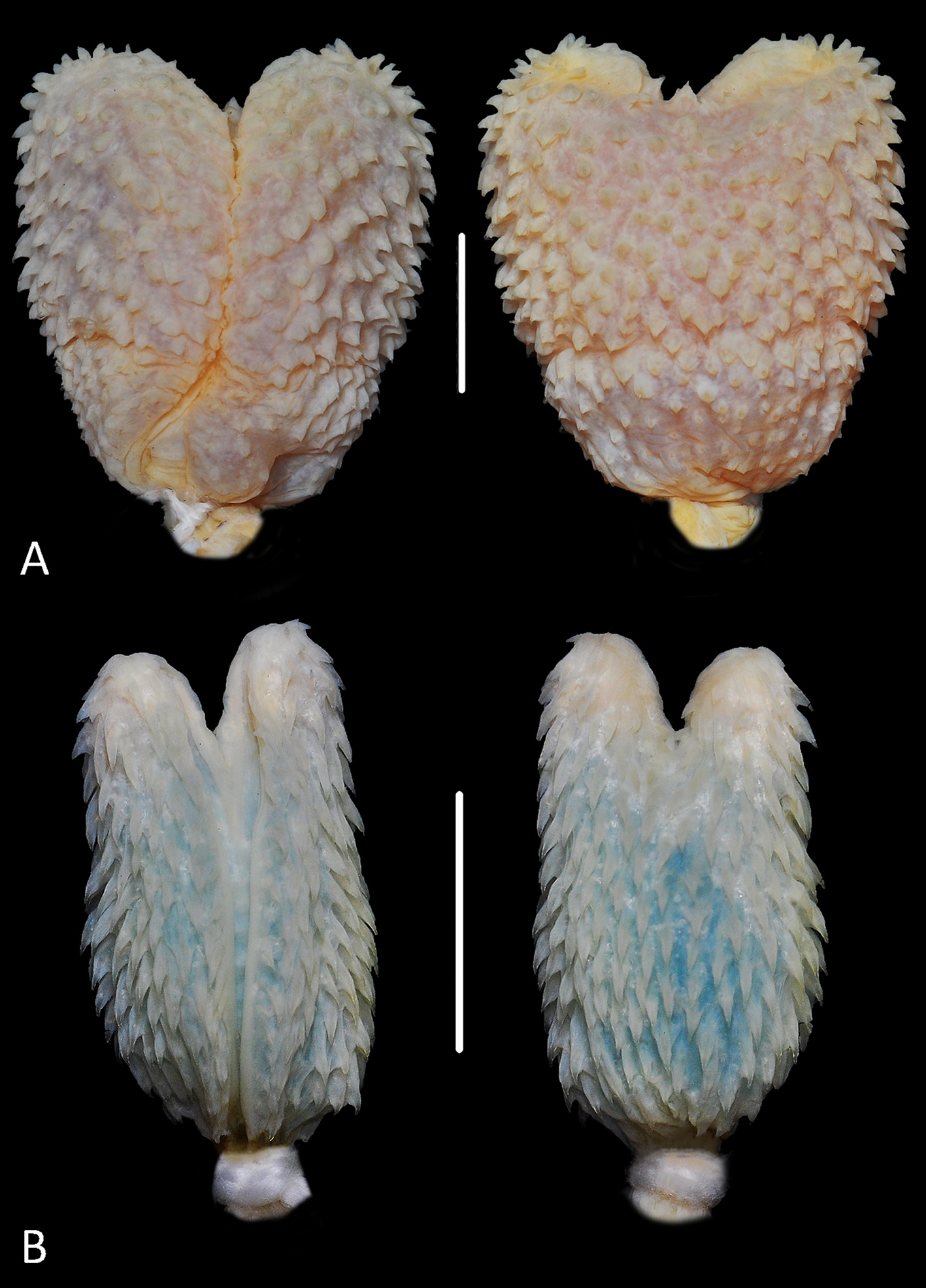
Hemipenial morphology (n = 2) ( Fig. 6B View FIGURE 6 ). Hemipenis, slightly bilobed, partially capitate and ornamented with calcified spines; deep sulcus spermaticus bifurcating on the second third of organ and running to apical region of lobes; sulcus spermaticus borders ornamented with spines along its entire extension; lobes short (approximately 30% of organ size), ornamented with spines smaller than those along hemipenial body, distributed in vertical rows; region between lobes with two rows of five diminutive spines; capitular crotch barely distinct on the sulcate and more evident on the asulcate face of hemipenis; body ornamented by few diminute spines; larger spines located on the central portion of capitulum, reducing in size towards lobes, and being less numerous on the sulcate face of organ; asulcate face covered ivy larger spines arranged in transverse rows; basal pocket weakly developed, without ornamentation and delimited by a central protuberance.
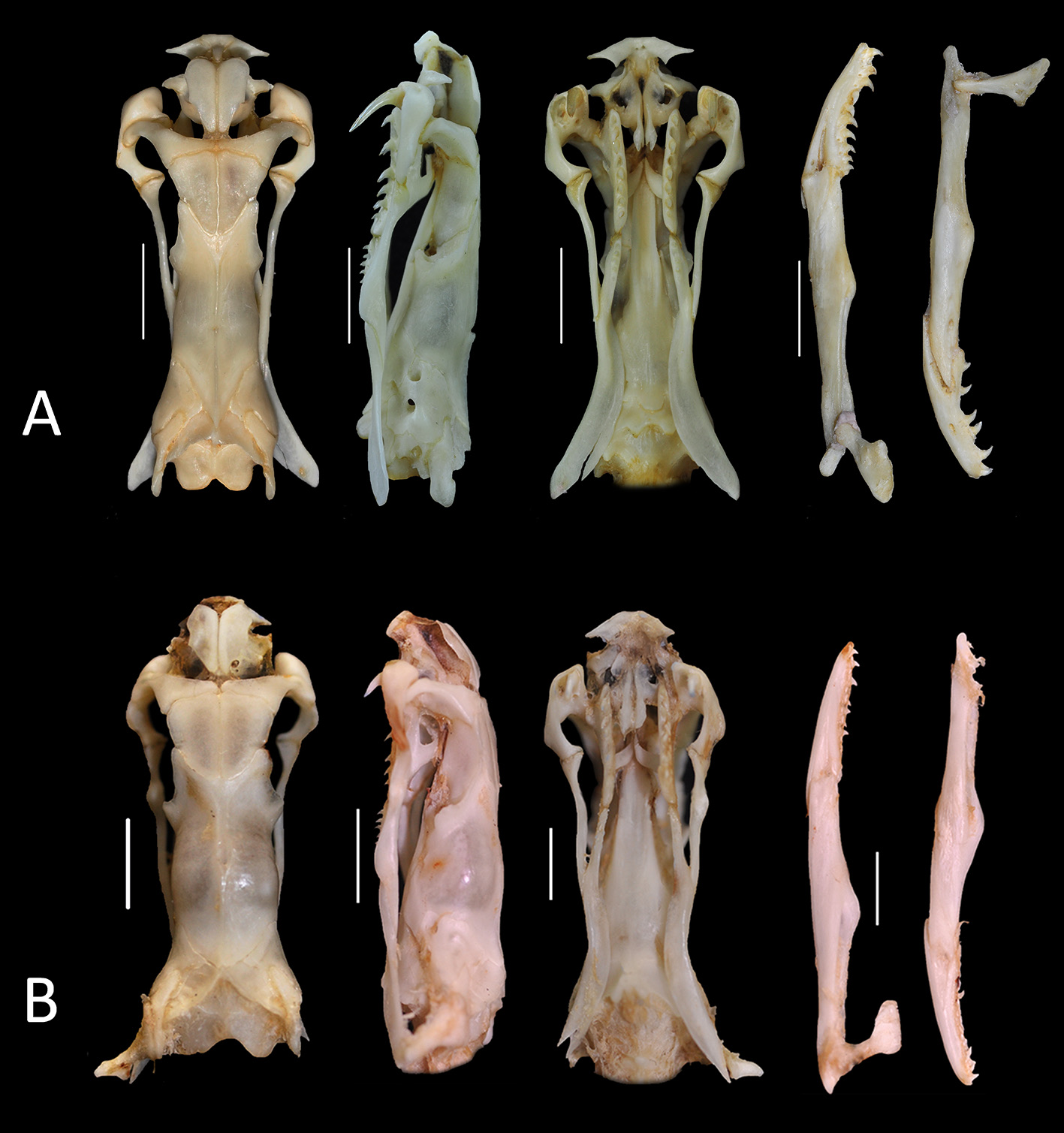
Skull morphology (n= 2) ( Fig. 7B View FIGURE 7 ). Premaxillar mostly located below nasal bones (45.41% of total skull width), with short lateral processus transversus with respect to premaxillary length; parietal wide (57.6% of length, 49.2% of width of the skull); proximal borders of parietal with outer edges forming posterior part of ocular orbit; medial parietal crest bifurcating on the proximal third, continuing orbital region; posterior borders rounded and do not surpass prootics limits; palatine (27.46% of skull width) with 7–8 teeth; pterygoid with 4–5 teeth; maxillary (19.87% of skull length) inclined toward ventral part of skull with proximal part wide and connected to prefrontal, contacting ectopterygoid on its middle third; venom fang short (52.98% length of maxillary), recurved posteriorally, inserted on the proximal ventral extremity of maxillary; dentary with 8–10 teeth; coronoid process not extensive.
Distribution ( Fig. 9 View FIGURE 9 ). Micrurus obscurus occurs from the central region of Bolivia (south bank of the Surutú River, near Santa Cruz de La Sierra) to the west, following the foothills of the Andes through Peru, Ecuador and Colombia (Vichada, Amanaven, right bank of the Orinoco River). In Brazil it occurs in the states of Acre (near the municipality of Candeias do Jamari) and Amazonas (municipality of Marabitanas), on the south bank of the Japurá River.
| MSNM |
Museo Civico di Storia Naturale di Milano |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Micrurus obscurus ( Jan, 1872 )
| Nascimento, Lywouty R. S., Silva Jr, Nelson J., Feitosa, Darlan T. & Prudente, Ana L. C. 2019 |
Micrurus spixii obscurus—
| Silva Jr. et al. 2016 |
Micrurus obscurus —
| Valencia 2016: 321 |
Micrurus obscurus —
| Wallach 2014: 451 |
Micrurus princeps —
| Wallach 2014: 451 |
Micrurus spixii obscurus — Campbell & Lamar 2004:229
| - Campbell & Lamar 2004: 229 |
Micrurus spixii princeps—
| Campbell & Lamar 2004 |
Micrurus obscurus —
| Harvey 2003: 22 |
Micrurus spixii obscurus—
| Schmidt 1953 |
Micrurus spixii princeps
| Schmidt 1953: 175 |
Micrurus spixii obscura
| Schmidt & Walker 1943: 294 |
Elaps princeps
| Boulenger 1905 |
Elaps heterozonus
| Peters 1881 |
Elaps corallinus var. obscura
| Jan in Jan & Sordelli 1872: 5 |