Xenotoca doadrioi, Domínguez-Domínguez, Bernal-Zuñiga
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4189.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:9BF8660A-4817-4EEA-853F-5856D1B8F6FA |
|
DOI |
https://doi.org/10.5281/zenodo.6057612 |
|
persistent identifier |
https://treatment.plazi.org/id/0393342A-FF9C-1526-FF4C-A7F95BBF9F88 |
|
treatment provided by |
Plazi |
|
scientific name |
Xenotoca doadrioi, Domínguez-Domínguez, Bernal-Zuñiga |
| status |
|
Xenotoca doadrioi, Domínguez-Domínguez, Bernal-Zuñiga , and Piller, sp. n.
( Figs. 5a View FIGURE 5. a , and Tables 2 View TABLE 2 to 6)
Http://zoobank.org/urn:lsid:zoobank.org:act:EFE95E0B-674F-4E7E-ADFA-971A5CCC0C20
Type material: Holotype. CPUM-9589, CPUM-T-41530, adult male 41 mm SL, Pond at San Sebastian village , North to Etzatlan, Jalisco, Mexico, Etzatlan endorheic drainage; 20°49’25``N and 104°7’10.8’’W, collected 17 June 2010 GoogleMaps . Paratypes. CPUM-5543, 22 specimens, CPUM-T 11929–11933, same data as holotype GoogleMaps . MNCN _ ICTIO 290.844 - 290.847 , 4 specimens, same data as holotype GoogleMaps . CNPE-IBUNAM20839, 4 specimens, same data as holotype GoogleMaps .
Diagnosis. Xenotoca doadrioi sp. n. is distinguished from the other species of the Xenotoca eiseni group and other Xenotoca species inhabiting the Pacific Coast drainages by the combination of the following characters (none unique to the species): the females have 14 dorsal rays versus 15 or 16 in X. melanosoma and 13 in X. lyonsi sp. n., 14 anal fin rays versus 15 or 16 in X. melanosoma , 12 pectoral fin rays versus 13 in Xenotoca eiseni , 8 caudal peduncle scales versus 9 in Xenotoca eiseni and X. melanosoma , 32 scales in a lateral series versus 31 in Xenotoca lyonsi n. sp. and 10 suparorbital pores versus 9 in Xenotoca eiseni and X. lyonsi ( Tables 2 View TABLE 2 to 4). Females of X. doadrioi show large caudal peduncle as is show by the x SL/EAHP = 3.8 versus x = 4.1–4.2 in X. eiseni and X. lyonsi and x SL/EDHP = 3.6 versus 3.9–4.2 in X. eiseni and X. lyonsi , large eye as is show by x HL/ED = 3 versus 3.5–3.7 in X. eiseni and X. lyonsi ( Table 5 View TABLE 5 ). Males have 14 dorsal rays versus 15 or 16 in X. melanosoma and 13 in X. lyonsi , 14 anal fin rays versus 15 or 16 in X. melanosoma , 12 pectoral rays versus 13 in Xenotoca eiseni , 8 caudal peduncle scales versus 9 in Xenotoca eiseni and X. melanosoma , 11 transversal scales versus 9 in X. lyonsi , 32 scales in a lateral series versus 31 in Xenotoca lyonsi and 10 suprorbital pores versus 9 in Xenotoca eiseni and X. lyonsi ( Tables 2 View TABLE 2 to 4). Poses a smaller head x HL/HH = 1.4 versus 1.1–1.2 in Xenotoca eiseni and X. lyonsi , the body is less high x SL/PPD = 4.7 versus 4–4.4 in Xenotoca eiseni and X. lyonsi and large caudal peduncle x SL/ EAHP = 3.8 versus 4–4.2 in Xenotoca eiseni and X. lyonsi and x SL/EDHP 3.8 versus 4.1–4.3 in Xenotoca eiseni and X. lyonsi ( Table 6 View TABLE 6 ).
Description. Frequency tables for each meristic character are shown in Tables 2 View TABLE 2 to 4. Xenotoca doadrioi has 12–14 dorsal rays, 13–15 anal rays, and 11–13 pectoral rays. Lateral scale series with 30–33, eight scales along the caudal peduncle, 9–11 transversal scales between dorsal and anal fin. The sensory pores of the lateral line system on the head are 8–9 opercular pores, 10–9 supraorbital pores, 2–4 mandibular pores and 4–5 preorbital pores ( Tables 2 View TABLE 2 to 4). The females are large than males; maximum known size for females is 47 mm, compared to 37 mm for males. Morphometrics measurements are show in tables 5 and 6. Body measurements are given in times the standard length, x = females/males. Body relatively deep, laterally compressed and elongated, anal fin inserted before the origin of the dorsal fin at same axis, PDD x = 2.6/2.4, PAD x = 5/5.2, DOAE x = 3.7/3.4, and DEAO x = 3.3/3.1, minimum body deep x = 6.4/5.8 being the females slightly deeper than males. Relative large caudal peduncle with respect to other species in the genus EAHP x = 3.8/3.8 and EDHP x = 3.6/3.8. Dorsal profile markedly convex with a marked hump at the nape in large specimens. Dorsal fin length long x = 6.5/5.6, being longer in males than in females. Head measurements are given in times the head length. The head is pointed, snout short, smaller than eye diameter, postorbital length HH x = 1.3/1.4, PrOL x = 5.4/6.1, PoOL = 2.2/2.5, eye relatively high, ED x = 3/3.6 being relatively bigger in females than in males. Mouth superior with the upper jaw slightly short than inferior.
Pigmentation pattern. When alive, the coloration varies with respect to the age and sex of the organism. Mature females display a general brownish coloration. Most mature females display dark blotches along the central part of the body, being bigger and conspicuous at the posterior half of the body; these blotches are formed by small black spots. Some scales show iridescent silver colorations in the body, being more evident in the postorbital and opercular region. Some females possess a dark stripe that runs along the middle part of the body, from the opercle to hypural plate. Scales are frequently rounded at their exterior margin by small black spots; a black blotch is present in the posteroventral region, between the pelvic and anal fins, which varies in depth and width ( Fig. 5a View FIGURE 5. a ). Juveniles have the same coloration as females, but as they reach ± 20 mm, they begin to differentiate to adult coloration. Males show the most colorful form of all Xenotoca species; this varies depending of the size and reproductive stage. In general, the caudal peduncle has an orange to almost red coloration combined with iridescent blue scales, the intensity and coverage of each color along the caudal peduncle is highly variable, some specimens show a blue or green to dark blue or green scales in the anterior part of the peduncle, the blue or green coloration extends to the origin of the dorsal fin, and also the intensity and coverage is highly variable, the caudal fin and frequently the anal and dorsal fin also have orange to red coloration in the base and sometimes the dorsal fin shown a dark coloration in the base. The portion of the body from the origin of dorsal and anal fin to pelvic or pectoral fin is pale in coloration, with gray to yellowish coloration, in the pre-ventral region. Orange to red coloration exists frequently and extends to the inferior part of the head. Just up to the pectoral fin there is a black blotch with iridescent scales that also is highly variable in intensity and size. There Is also blue iridescent coloration in the opercle and in some scales along the body ( Fig. 5a View FIGURE 5. a ). The coloration of preserved specimens varies with respect to fixation and time since fixation, but in general, female specimens preserved in 5% formalin possess clear brownish coloration. The blotches are less evident along the body, in larger females they are still present. Numerous dark small spots are found in all the upper half of the body. A silver stripe is present along the middle part of the body, being more evident in the posterior half. The dark blotch in the posteroventral region is still evident. The opercle shows a silver coloration. Males lose all coloration when preserved. The peduncle and pre-ventral region show a clearer brownish coloration. The rest of the body shown a more brownish dark coloration with numerous black spots distributed along the upper half of the body. Fins clear and unpigmented, a few specimens still show a dark blotch up to the pectoral fin and the scales are rounded by a numerous black spots.
Sexual dimorphism. As is the case with other members in the subfamily Goodeinae , sexual dimorphism is substantial, with males showing a reduced length on the first five to seven anal-fin rays ( Hubbs & Turner 1939). Females are large than males. The base of the anal, dorsal and pectoral fins are larger in males than in females, (females/males) as show by the SL/DFL x = 6.5/5.5, SL/AFL x = 9.7/8.6 and SL/PFL x = 17.1/15.2. Males are deeper than females SL/BLD x = 5.8/6.4, SL/PDD x = 2.4/2.6 and SL/DOAE x = 3.4/3.7. The males have smaller eyes (females/males) HL/ED x = 3/3.6 ( Tables 5 View TABLE 5 and 6 View TABLE 6 ). The most evident dimorphism is in coloration, with males much more colorful than females ( Fig. 5a View FIGURE 5. a ).
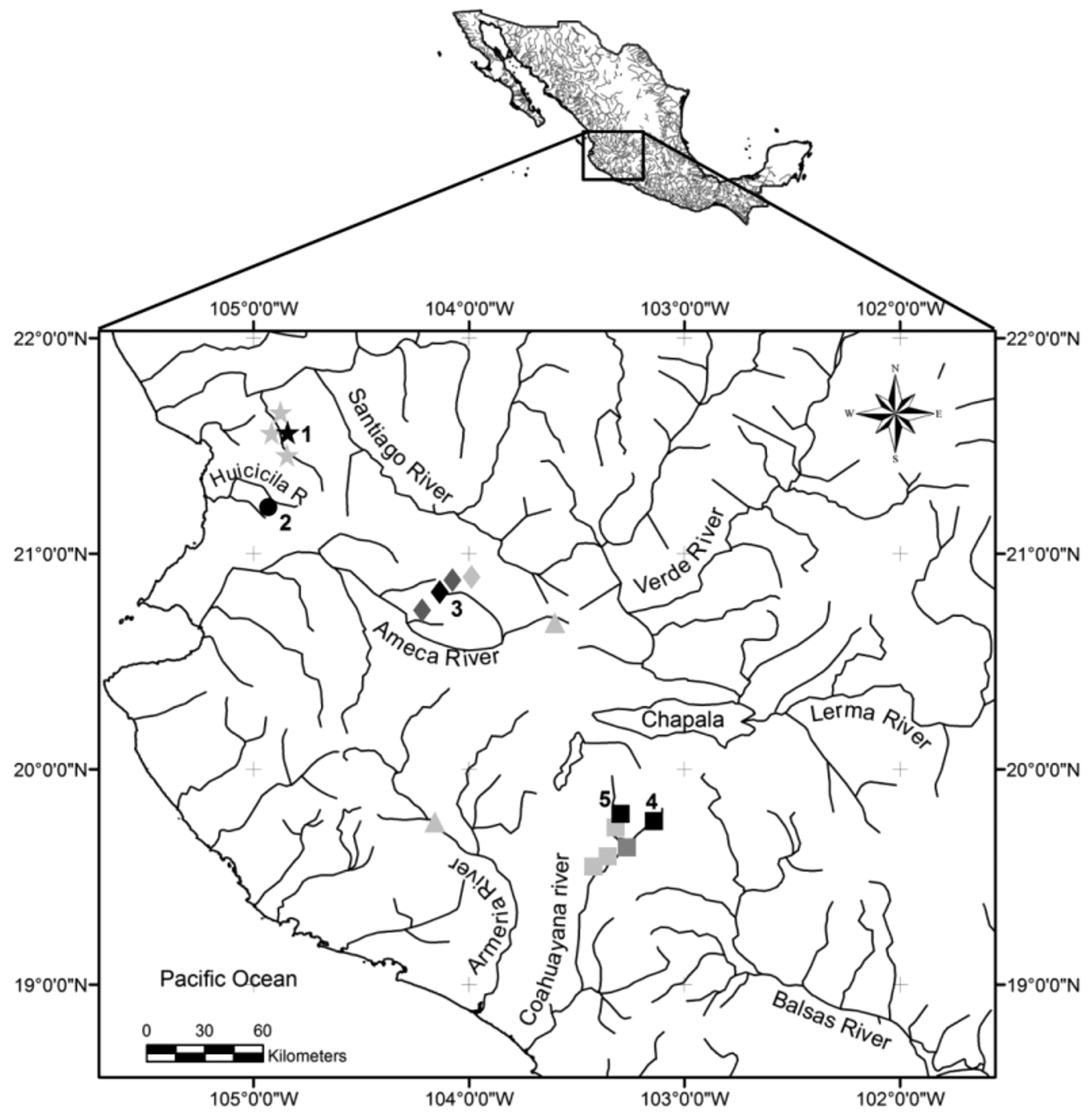
Distribution. The species is endemic to the endorheic region of Etzatlan, in the state of Jalisco, Mexico ( Fig. 1 View FIGURE 1 ). The type locality is a small and permanent pond just in the east end of the Hacienda San Sebastian, with around 6,000 m 2 fed by a spring (20°49’25’’ N, 104°7’10.8’’ W). Other known locations in the area are El Moloya spring, Estancia de Ayoles reservoir, Oconahua Dam around 3km west of Oconahua village, and the highly perturbed and seasonally affected streams along the federal road number 4, between Etzatlan and San Marcos Village, known as arroyo San Marcos and arroyo de la Granja Sahuaripa, but the last two locations have not yielded specimens since 2006, and in an extensive survey in 2015, these localities were found to be totally dry or full of Pseudoxiphophorus bimaculatus ( Heckel 1848) when water was present.
Etymology. The name of the species, an adjective, is derived from the name of the prestigious ichthyologist Dr. Ignacio Doadrio , Museo Nacional de Ciencias Naturales, Spain, who has strongly contributed to the study and knowledge of Mesoamerican fish diversity.
Habitat and ecology. This species seems to be highly adaptable to variable habitat conditions. At the type locality, the species inhabits an area with turbid water, and was collected in a shallow water no more than 1.5 m deep. The pond is no more than 3 meters at its deepest part; the bottom is comprised of mud and gravel, and no water plants are present. Other fish species collected in the area were Xenotoca melanosoma , Goodea atripinnis Jordan 1880 , Poeciliopsis infans ( Woolman 1894) and the introduced Xiphophorus variatus ( Meek 1904) and Oreochromis sp. Historically, other species reported from this pond include Algansea amecae Pérez-Rodríguez, et al. 2009 , Moxostoma austrinum Bean 1880 , and Allotoca maculata Smith & Miller 1980 , but all of these species have not been collected in the area since 1970. In the El Moloya Spring, the species inhabits clear water with gravel to muddy bottom and water plants and this pond is used as a swimming pool. Other species inhabiting this pond are X. melanosoma , Zoogoneticus purepechus Domínguez-Domínguez et al. 2008a , Ameca splendens Miller and Fitzsimons 1971 , G. atripinnis , P. infans , and the introduced Oreochromis sp. In Oconahua Dam, the water is turbid and contains a muddy bottom and with few water plants. Other species collected include X. melanosoma , G. atripinnis , P. infans , as well as the introduced Lepomis macrochirus Rafinesque 1818 , and Cyprinus carpio Linnaeus 1758 . The San Marcos stream is a seasonally fluctuating stream that is dry for most of the year, but when water is present the surface of the stream is totally cover with Eichhornia crassipes Martius, Thypa sp., and Cyperus sp. The water at this site is highly polluted by organic matter and is turbid, whereas the Sahuaripa stream is an irrigation channel totally modified and fed by a water pump; in 1999 and 2002, the species collected in both places included X. melanosoma , Allotoca sp., G. atripinnis , P. infans , and Oreochromis sp. For the 2006 survey, Allotoca sp. was not collected; in the 2015 survey only Pseudoxiphophorus bimaculatus was found. Nothing is known about its biology in nature.
Conservation. Xenotoca doadrioi is known only from small springs and a dam in areas highly impacted by human activities that have been strongly modified for irrigation. It has been extirpated from more than 50% of the known historical localities ( Pedraza-Marrón 2011). This species is found in small numbers in the three localities where it presently occurs, and these localities are under the influence of substantial irrigation pressure for agriculture. Introduced fish species pose a significant risk for the long-term survival of this species. It is recommended that X. doadrio be considered a species in danger of extinction.
| MNCN |
Museo Nacional de Ciencias Naturales |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |