Richerius marqueti, Guinot & de Mazancourt, 2020
|
publication ID |
https://doi.org/10.5852/ejt.2020.671 |
|
publication LSID |
lsid:zoobank.org:pub:9EF19154-D2FE-4009-985C-1EB62CC9ACB0 |
|
DOI |
https://doi.org/10.5281/zenodo.7433864 |
|
persistent identifier |
https://treatment.plazi.org/id/0393774D-400E-B22A-6611-1BB8FBFFFDFC |
|
treatment provided by |
Valdenar |
|
scientific name |
Richerius marqueti |
| status |
gen. et sp. nov. |
Richerius marqueti View in CoL gen. et sp. nov.
urn:lsid:zoobank.org:act:
Figs 1–5 View Fig View Fig View Fig View Fig View Fig
Etymology
The species name is in honour of Gérard Marquet, who made extensive collections of freshwater crustaceans for more than 30 years in the Indo-Pacific islands and in particular in New Caledonia where he collected the new species here described, for his friendship and his constant enthusiasm in the field as well as in the laboratory.
Type material
Holotype ( Figs 1–2 View Fig View Fig , 4 View Fig )
NEW CALEDONIA • ♂, 4.9 × 5.0 mm; South Province, Bourail township, Bouïrou village, Pouéo River, tributary of the Néra ; 21º26.326ʹ S, 165º31.909ʹ E; 180 m a.s.l.; 28 Sep. 2016; Valentin de Mazancourt and Gérard Marquet leg.; DNA voucher: CA2188; GenBank: MT364999 View Materials ; MNHN-IU-2014-21500 GoogleMaps .
Paratypes
NEW CALEDONIA – South Province • 1 ♂, 4.5 × 4.7 mm; same collection data as for holotype; DNA voucher: CA2189; GenBank: MT365000 View Materials ; MNHN-IU-2014-21504 GoogleMaps • 1 immature; South Province, Bourail township, Bouïrou village, Pouéo River, tributary of the Néra, st. HYNC 799 ; 21º26.310ʹ S, 165º31.917ʹ E; 186 m a.s.l.; 17 Nov. 2017; “Our Planet Reviewed”, Hydrobio exped; Valentin de Mazancourt and Nicolas Charpin leg.; MNHN-IU-2014-21188 GoogleMaps • 1 ♀, 5.0 × 5.4 mm; same collection data as for preceding; DNA voucher: CA2185; GenBank: MT364996 View Materials ; MNHN-IU-2014-21505 GoogleMaps • 1 ♂, 4.8 × 5.2 mm; same collection data as for preceding; DNA voucher: CA2186; GenBank: MT364997 View Materials ; MNHN-IU-2014-21506 GoogleMaps . – North Province • 1 ovigerous ♀, 7.0 × 7.1 mm; Houaïlou township, Creek stream, tributary of the Böua at level of the Néaoua dam, st. HYNC 1823 ; 21º21.890ʹ S 165º32.683ʹ E; 476 m a.s.l.; 9 Oct. 2017; “Our Planet Reviewed”, Hydrobio exped,; Nicolas Charpin leg.; DNA voucher: CA2197; GenBank: MT365001 View Materials ; MNHN-IU-2014-21501 GoogleMaps • 1 ♂, 4.1 × 4.0 mm; same collection data as for preceding; DNA voucher: CA2198; GenBank: MT365002 View Materials ; MNHN-IU-2014-21502 GoogleMaps • 3 immature specs; same collection data as for preceding; DNA voucher: CA2187; GenBak: MT364998 View Materials ; MNHN-IU-2014-21503 GoogleMaps .
Description
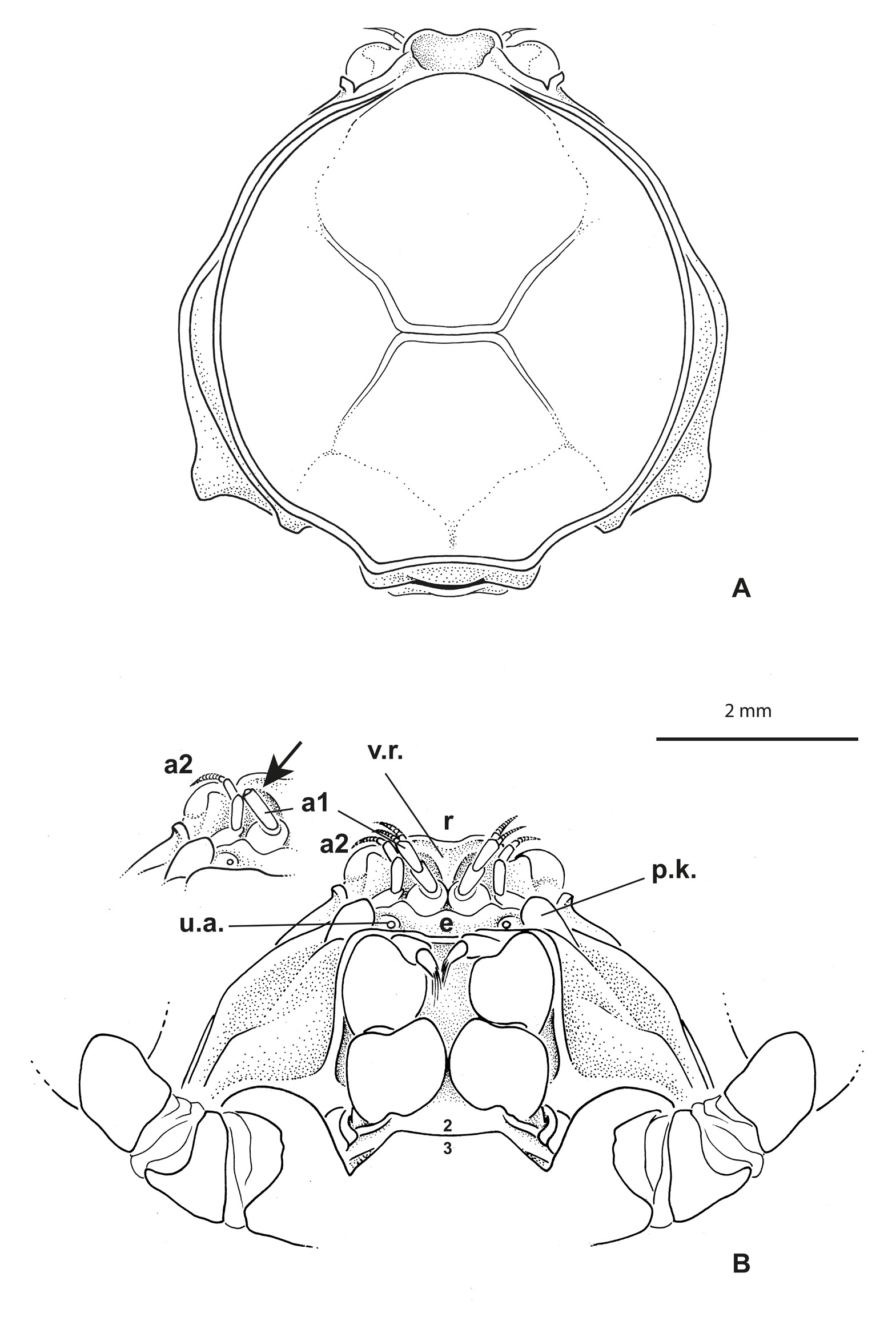
Carapace ( Figs 1A View Fig , 5B View Fig ) approximately circular to oval, slightly broader than long; dorsal carapace surface slightly concave, not strongly outlined by grooves, only with well defined gastrocardiac and thoracic grooves, approaching but not reaching antero- and posterolateral margins, respectively; no row of setae along lateral margins, except for some regularly mid-spaced setae in males; carapace angles not well marked. Anterolateral margin entire, without crenulations, lobes or teeth. Branchiostegite only weakly visible dorsally.
Eyes visible dorsally. Antennules ( Fig. 1B View Fig ) obliquely folded along hollowed ventral parts of rostrum and entirely hidden dorsally. Antennae well separated from antennules, at least at their bases; urinary article at level of epistome. Rostrum ( Figs 1 View Fig , 4 View Fig , 5 View Fig B–D) broadly rounded, spade-shaped, barely deflexed; dorsal surface spatulate with marked depression; carapace rim as a small ridge continuous across behind rostrum. Proepistome represented by triangular ventral expansion of rostrum ( Fig. 1B View Fig ). Epistome moderately developed; anterior margin undulated. Lower orbital margin with one conspicuous knob, not visible dorsally. Pterygostomial regions with setae, distinctly separated from subhepatic area by marked ridge. Mxp3 moderately gaping, broad; merus and ischium broad, short; midlength of merus slightly longer than that of ischium ( Figs 1B View Fig , 2B View Fig ). Sternum/pterygostome junction substantially developed due to extension of sternite 4. Milne-Edwards openings separated from chelipeds.
Male chelipeds much stouter than walking legs, particularly in large males ( Fig. 4 View Fig ); merus and carpus with stiff, regularly spaced setae; propodus very inflated, covered with long soft setae partially extending on fingers; fingers with finely denticulate cutting edges, not gaping. Female chelipeds narrow, propodus moderately inflated, devoid of long setae; fingers proportionally rather long, with with finely denticulate cutting edges completely joined. Walking legs proportionally rather long, with margins bearing stiff, regularly spaced, scattered setae; dactyli slender but not distinctly longer than respective propodi, smoothly curved, setose, without teeth, ending in pointed tip.
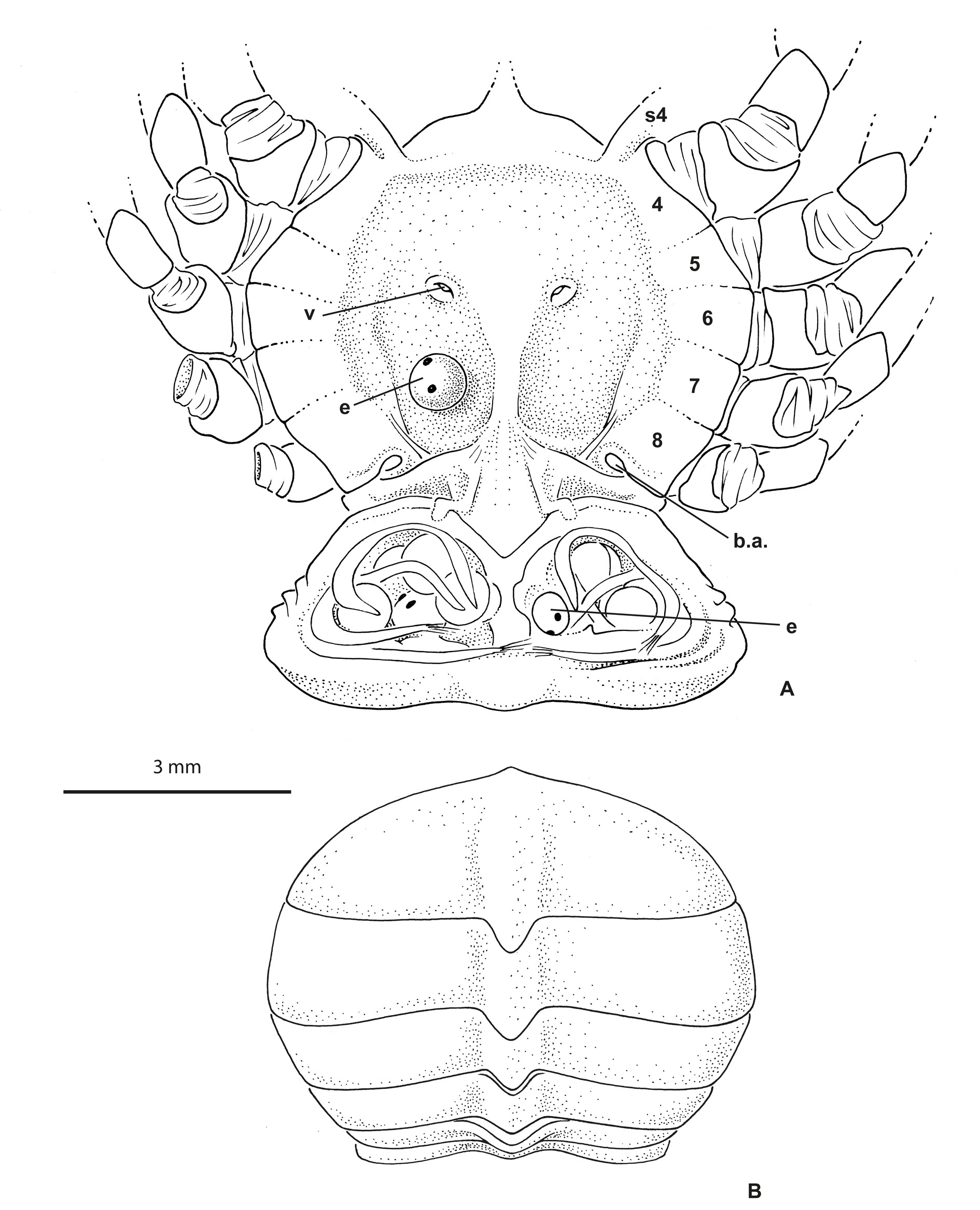
Thoracic sternum of male ( Fig. 2A View Fig ) with sternites 4–8 considerably enlarged, with suture 4/5 to 7/8 laterally confined. Sternites 1 and 2 not separated by suture but their demarcation visible on lateral margins; sternite 2 separated from sternite 3 by complete suture; sternite 3 as narrow pentagonal band; suture 3/4 tiny, only lateral, just at level of extension of sternite 4. No longitudinal line. Paired branchiosternal canal apertures located posterolaterally on sternite 8, concealed by pleon ( Fig. 3A View Fig ).
Sternopleonal cavity of male short, triangular, deep ( Fig. 2C View Fig ). Pleons in both sexes ( Figs 2A View Fig , 3B View Fig ) without fused somites, except 6 fused to telson, thus five somites plus pleotelson. Male pleon widely triangular, regularly widening from base to pleotelson; pleotelson widely triangular, somewhat trilobed, with intercalated plates partially delineated, salient and visible laterally at base, partially included. Pressbutton on lateral side of sternopleonal cavity ( Fig. 2C View Fig ). Male gonopore sternal.
Female pleon ( Fig. 3 View Fig A–B) oval to expanded in ovigerous females. Female pleopods 2–5 biramous. Ovigerous females with very few and large eggs measuring 0.96 mm in diameter ( Fig. 3A View Fig ). Vulvae located on undivided portion of thoracic sternum corresponding to sternite 6, thus not deplaced anteriorly. First gonopods stout, practically filling most of sternopleonal cavity ( Fig. 2C View Fig ). G1 curved at base, otherwise with little curvature, with terminal portion bearing fine setae, terminating in one lobe, without corneous process ( Fig. 2D View Fig ).
Colour
In life ( Fig. 5 View Fig B–D), the colour is overall brown, mottled with yellowish dots.
Distribution
Richerius marqueti gen. et sp. nov. is exclusively known from freshwaters in small flowing streams where it lives among the aquatic vegetation ( Fig. 5A View Fig ). It is found at an altitude of up 500 m, therefore further from the sea than Odiomaris pilosus (that is usually encountered in the lower course of rivers). By comparison, Amarinus lacustris (Chilton, 1882) has been reported from New Zealand freshwaters from Lord Howe Island at altitudes between 61–92 m ( Etheridge 1889) and even about 800–900 m ( Chilton 1915; Holthuis 1968); A. angelicus Holthuis, 1968 , from the central mountain range of Papua New Guinea, was collected in pure freshwater of a watercress swamp at an altitude of 1600 m ( Holthuis 1968, 1982).
Remarks
The subfamily Odiomarinae was erected by Guinot (2011a) to receive two genera of the family Hymenosomatidae characterised by the presence on the male pleon of intercalated platelets, either articulated and moveable ( Guinot 2011a: fig. 2) or relatively less well-demarcated: Odiomaris Ng & Richer de Forges, 1996 , endemic to New Caledonia, and Amarinu (at least pro parte), mostly from fresh and estuarine waters of the Indo-West Pacific region. Richeriu s gen. nov. shows several plesiomorphic characters that include the male and female pleons without fused somites (except for pleotelson), thus consisting of six elements (namely the maximum of somites existing in Hymenosomatidae ), the prominent, partially demarcated intercalated platelets, the thoracic sternum with the anterior sternites forming a small produced plate, the vulvae not anteriorly displaced, the G1 only gently curved and simple. Richerius gen. nov. can be assigned to the Odiomarinae .
Odiomaris is known from two species: the type species Elamena pilosa A. Milne-Edwards, 1873 , referred to as Halicarcinus White, 1846 by Holthuis (1968), as Amarinus by Lucas (1980) then as Odiomaris by Ng & Richer de Forges (1996), mainly freshwater but also euryhaline; and O. estuarius Davie & Richer de Forges, 1996 , exclusively brackish. An important difference between these two species is the rostrum: it is lowered ventrally as a triangular, V-shaped projection in O. pilosus so as to be positioned between the antennules ( Fig. 7A View Fig ) (A. Milne-Edwards 1873: pl. 18, fig. 6a, as Elamene pilosa ; Ng & Richer de Forges 1996: fig. 6c–d), whereas it is spatulate and does not extend to form a ventral projection between the antennules in O. estuarius ( Fig. 8 View Fig A–B) ( Davie & Richer de Forges 1996: fig. 1a–b).
Richerius gen. nov. shares with Odiomaris the same arrangement of the cephalic appendices. But its rostrum is spatulate without folding down ventrally ( Figs 1 View Fig , 4 View Fig , 5D View Fig ), instead of being lowered ventrally as a V-shaped projection located between the antennules as in Odiomaris pilosus ( Fig. 7A View Fig ). In Richerius gen. nov. ( Fig. 1B View Fig ), the proepistome is represented by a ventral expansion of the rostrum; therefore, the antennules are located along the proepistome, whereas in Odiomaris pilosus the antennules are obliquely folded in a fossa hollowed all along with the lateral parts of the V-shaped rostrum and the eye. Richerius marqueti gen. et sp. nov. actually has a rostrum and proepistome more similar to those of O. estuarius ( Fig. 8 View Fig ) ( Davie & Richer de Forges 1996: fig. 1) than to those of O. pilosus . In fact, the genus-level differences between Richerius gen. nov. and Odiomaris remain unclear. Actually, O. estuarius seems morphologically closer to Richerius gen. nov. than to O. pilosus , at least in some characteristics. A possible transfer of O. estuarius to Richerius gen. nov. has been considered but needs to be decided later, especially when the status of Odiomaris aff. pilosus is resolved (see below, ‘Remarks on Odiomaris aff. pilosus from the Iouanga River and two other streams’).
Along the lower orbital margin there are two salient knobs in both species of Odiomaris , both prominent in dorsal view in O. pilosus (A. Milne-Edwards 1873: pl. 18, fig. 6a; Ng & Richer de Forges 1996: fig. 6a, d, not figured in fig. 6c; Guinot & Richer de Forges 1997: figs 1a, c, 2c), but shorter and not dorsally visible in O. estuarius ( Davie & Richer de Forges 1996: fig. 1a; not figured in fig. 1b). In contrast, there is only one, smaller knob in Richerius gen. nov. ( Fig. 1B View Fig ). The cheliped palm of R. marqueti gen. et sp. nov. is covered with long, flexible setae ( Fig. 5 View Fig C–D), instead of numerous spinules (or stiff setae) in O. pilosus ( Fig. 6 View Fig ) (A. Milne-Edwards, 1873: pl. 18, fig. 6d; Ng & Richer de Forges 1996: fig. 6f), and sparse, short setae in O. estuarius ( Fig. 8 View Fig ). In fact, the whole body (including the margins of the rostrum) and legs of O. pilosus are covered with spiniform, stiff setae that give a bristle appearance ( Ng & Richer de Forges 1996: figs 5a–b, 6a, d; Guinot & Richer de Forges 1997: fig. 1a), whereas O. estuarius has a carapace with soft, short setae and legs with longer setae ( Davie & Richer de Forges 1996: fig. 1a). The male cheliped fingers are practically joining in R. marqueti gen. et sp. nov. ( Fig. 5C View Fig ), but with a broad proximal gap in O. pilosus ( Fig. 6 View Fig A–B) (A. Milne-Edwards 1873: 322, pl. 18, fig. 6, 6d, as Elamene pilosa ; Ng & Richer de Forges 1996: fig. 6f); in O. estuarius the chelae are narrower, with long fingers without marked gap ( Fig. 8 View Fig A–B) ( Davie & Richer de Forges 1996: fig. 1). The grooves on the carapace dorsal surface are very distinct in O. pilosus , well marked in R. marqueti gen. et sp. nov., whereas almost indiscernible in O. estuarius . The sterno-pleonal cavity is short in Richerius gen. nov. and in Odiomaris , and accordingly the pleon also. The male pleon is as a narrow triangle, with a long pleotelson in both Odiomaris species (A. Milne-Edwards 1873: pl. 18, fig. 6b, as Elamene pilosa ; Ng & Richer de Forges 1996: fig. 7a–b; Guinot & Richer de Forges 1997: fig. 2a: O. pilosus ; Davie & Richer de Forges 1996: fig. 2c: O. estuarius ), markedly wider and with a much shorter pleotelson in R. marqueti gen. et sp. nov. ( Fig. 2A View Fig ). The intercalated platelets, which are completely demarcated and moveable in O. pilosus ( Guinot 2011a: fig. 2), are only partially delineated in R. marqueti gen. et sp. nov., and not detached in O. estuarius . The female pleon is rather similar in Richerius gen. nov. and Odiomaris , with five somites plus pleotelson. The G1 of Richerius gen. nov. ( Fig. 2D View Fig ) is curved at the base, otherwise with little curvature, with a terminal portion bearing fine setae and ending in one lobe without corneous process, whereas the G1 of Odiomaris is characterised by two distinct distal processes, a longer corneous process and a shorter lobular elongation of the stem ( Fig. 7B View Fig : O. pilosus ) ( Ng & Richer de Forges 1996: fig. 7c– d: O. pilosus ; Davie & Richer de Forges 1996: fig. 2c: O. estuarius ). O. estuarius is a much smaller species than O. pilosus , and R. marqueti gen. et sp. nov. seems to be nearly as small as O. estuarius .
The main differences to distinguish the two genera Odiomaris and Amarinus stated by Davie & Richer de Forges (1996: 259) were: 1) in Odiomaris , the G1 (see Fig. 7B View Fig ) more slender, with two distinct distal processes, a longer corneous process and a shorter lobular elongation of the stem than in Amarinus ; 2) in Odiomaris , the elongated triangular telson of the male pleon is significantly longer than wide at base, whereas in Amarinus the telson is more or less rounded and short, being much wider than long.
The genus Amarinus Lucas, 1980 ( type species by original designation: Elamena lacustris Chilton, 1882 ) is known from more than ten species. They all inhabit low salinity environments, from brackish habitats to pure freshwater waters permanently (streams, lakes, swamps), and have a large distribution ( New Zealand, Australia, Indonesia, the Philippines, Papua New Guinea). Cases where Amarinus has been reported from New Caledonia, as in Chuang & Ng (1994: 87, 90, table 1, under A. pilosus ) and erroneously as in Guinot (2011a: 23), are attributable to the fact that the species pilosus was previously associated with the genus Amarinus (see Lucas 1980) until Ng & Richer de Forges (1996) made it the type species of their new genus Odiomaris . So far, no species of Amarinu s has actually been reported in New Caledonia. Suspecting that our new species might belong to the genus Amarinus , we therefore carefully compared Richerius gen. nov. to Amarinus , and in particular to its type species, A. lacustris , another freshwater hymenosomatid.
Richerius marqueti gen. et sp. nov. can be distinguished from Amarinus lacustris by: the wide male pleon, with prominent marks corresponding to intercalated platelets ( Fig. 2A View Fig ) (versus narrow and without intercalated platelets in A. lacustris , see Melrose 1975: fig. 42g; Lucas 1980: fig. 7b); the G1 rather narrow and with one distal lobe without corneous process ( Fig. 2D View Fig ) (versus stout and without lobes, see Melrose 1975: fig. 42h–i; Lucas 1980: fig. 10e); and by the arrangement of antennules and antennae.
In providing a key of the Southeast Asian hymenosomatids, Ng & Chuang (1996: 3–5, 6–12) have shown the presence of several groups of species within Amarinus , suggesting that the genus could be paraphyletic. Today, another problem arises, especially regarding the antennular morphology. Melrose (1975: 84, 87, figs 41–42, as Halicarcinus lacustris ), who has thoroughly studied the type species A. lacustris , confined to lakes and non-tidal rivers of New Zealand and southeastern Australia, states that the antennules are “small, not visible dorsally when folded”. Her figure 41d actually seems to show a folded antennule, only with the broad basal article and the second cylindrical article, without the short, supposedly folded flagellum being seen [the frontal view of fig. 41e in Melrose (1975) shows a complete, unfolded antennule]. Our examination of Amarinus lacustris , on the other hand, reveals that both antennules and antennae are inserted very closely together at their bases and remain parallel, with both flagella visible dorsally, as represented by Lucas (1980: fig. 1d) for a generalised hymenosomatid or by Melrose (1975: fig. 43c) for Halicarinus tongi Melrose, 1975 . In fact, if it is possible for the antennule of A. lacustris to fold itself down [but not obliquely along the rostrum as in Odiomaris ( Fig. 7A View Fig ), see A. Milne-Edwards 1873: pl. 18, fig. 6a], this does not correspond to the resting position. In the seven specimens of A. lacustris examined, the antennule and antenna are very closely inserted and remain parallel, both being stretched forward; the unfolded flagellum is visible dorsally in all individuals. In any event, the disposition of the antennule and antenna differs from that Odiomaris ( Fig. 7A View Fig : O. pilosus ) ( Ng & Richer de Forges 1996: fig. 6c–d: O. pilosus ).
A picture of the cephalic region in ventral view of Amarinus angelicus ( Holthuis 1968: 114, fig. 2b) shows an antennule “entirely hidden below the rostrum”, obliquely folded, which does not seem to correspond to the condition of A. lacustris . This character deserves to be reviewed for all species of Amarinus , and the allocation of Amarinus (including the type species and other included species) to the Odiomarinae needs further investigation. In the new species described here, Richerius marqueti gen. et sp. nov. ( Fig. 1B View Fig ), the antennule and antenna are separated at least at their insertion site, and only the antennal flagellum is visible dorsally; thus it shares an arrangement substantially similar to that of Odiomaris .
It was the study of spermatozoa carried out first in the two species of Odiomaris , O. pilosus and O. estuarius , by Richer de Forges et al. (1997) and later in Elamena vesca by Jamieson & Tudge (2000) that showed the very particular nature of the hymenosomatid sperm within the Brachyura . Indeed, they are distinguished by at least nine major characteristics from those of all the other Brachyura taxa studied, particularly the groups with which they have been associated, the Majoidea Samouelle, 1819 and the Thoracotremata Guinot, 1977. The ‘hymenosomatid-type of spermatozoon’ is unique within Brachyura ( Tudge et al. 2014). The highly developed projection of the acrosome from the nucleus in hymenosomatid spermatozoon recalls the totally emergent acrosome of Podotremata Guinot, 1977 and may represent the plesiomorphic condition in the Eubrachyura Saint Laurent, 1980 ( Guinot 2011a, 2011b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
SuperFamily |
Hymenosomatoidea |
|
Family |
|
|
SubFamily |
Odiomarinae |
|
Genus |