Cophixalus kulakula, Hoskin, Conrad J. & Aland, Kieran, 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.206707 |
|
DOI |
https://doi.org/10.5281/zenodo.6193250 |
|
persistent identifier |
https://treatment.plazi.org/id/0394566B-FFCB-FF29-F8EA-EECD9B01B2E8 |
|
treatment provided by |
Plazi |
|
scientific name |
Cophixalus kulakula |
| status |
sp. nov. |
Cophixalus kulakula View in CoL sp. nov.
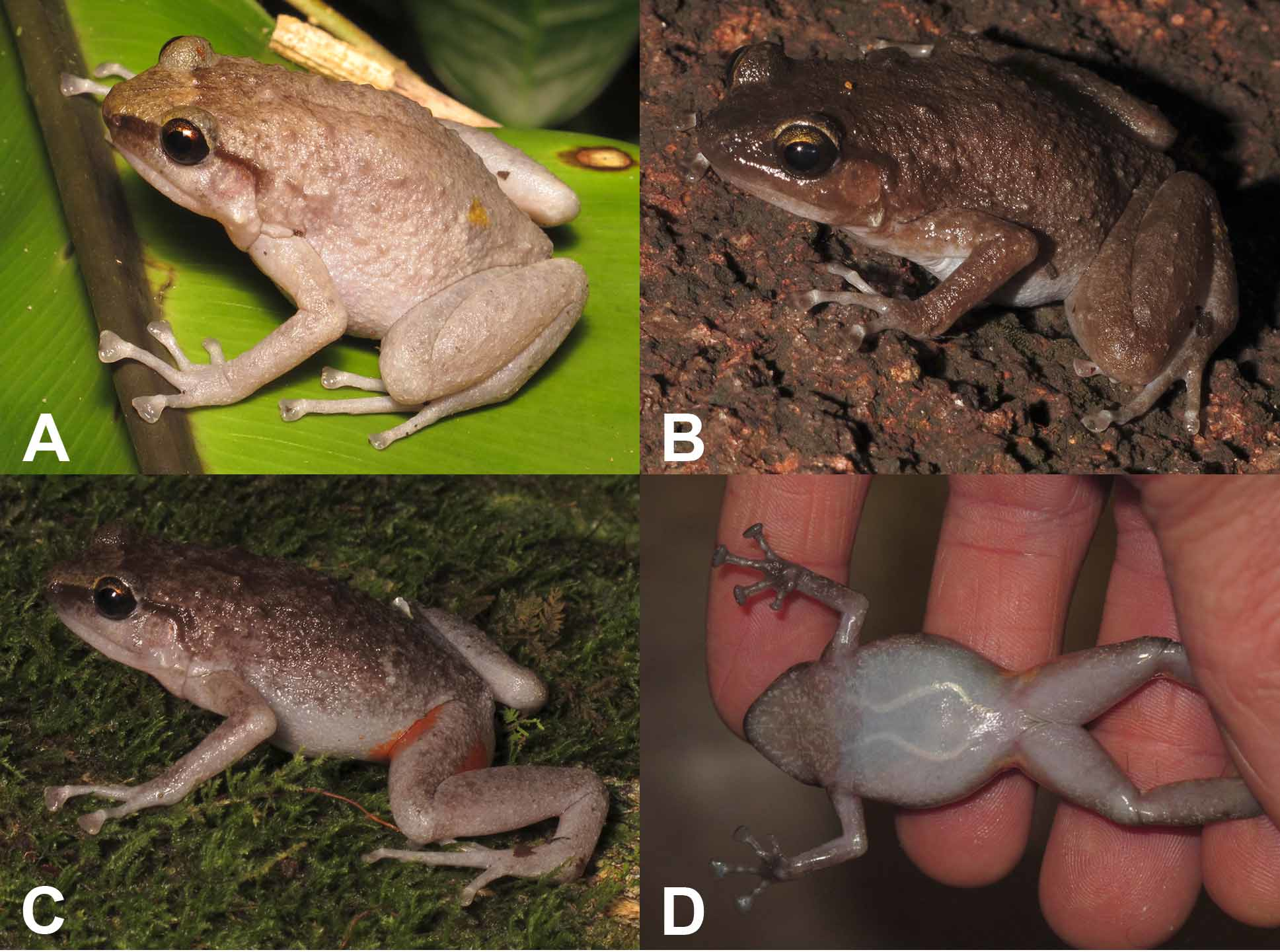
Kutini Boulder-frog ( Fig. 3 View FIGURE 3 )
Material examined. Holotype: QMJ88540, female, boulder field hill approx. 5 km NW of Mt Tozer summit (12°42′37S, 143°10′22″E, elevation 180 m), Cape York Peninsula, north-east Queensland, C. J. Hoskin and K. Aland, 13 April 2010. Paratypes: QMJ88536 (male), QMJ88537 (male), QMJ88538 (juvenile), QMJ88539 (female), collection details as for holotype; QMJ88503 (female), QMJ88504 (male), gully on eastern side of Mt Tozer (12°45′46S, 143°13′26″E, elevation 231 m), K. Aland, 20 February 2009; QMJ88541 (female), QMJ88542 (female), QMJ88543 (male), QMJ88544 (male), boulder field on western slopes of Mt Tozer uplands (12°43′46S, 143°11′0 7″E, elevation 170 m), K. Aland and C. J. Hoskin, 13 April 2010. Additional material: An additional two individuals from this latter site were measured in the field.
Diagnosis. A medium sized frog with long, slender fingers and large, obviously truncated finger pads. Cophixalus kulakula sp. nov. can be distinguished from its congeners by a combination of the following characters: large size (SVL: 40–48 mm, average 43 mm), red/orange in groin and on posterior thigh (but not in axilla), fairly uniform pale or brown dorsum and flanks. The mating call is also distinct from all Cophixalus for which the call is known, in being a short, wavering ‘bleat’.
Etymology. From kul’a kul’a, meaning ‘rocky place’ in Kuuku Ya’u, a language of the Sandbeach People of Eastern Cape York. This epithet was suggested by Mr Ronald Giblet, custodian of the Kutini clan estate in which the first specimens were discovered. The species epithet is used as a noun in apposition.
Measurements of holotype. QMJ88540, female; SVL 43.0 mm; TL 21.5 mm; FL 10.5; HW 15.8 mm; HL 11.7 mm; ED 3.7 mm; EN 3.4 mm; IN 3.6 mm; 3DW 2.9 mm; 3FL 8.1 mm; 4TL 11.3 mm.
Description of type series. Data presented as range followed by mean in brackets. Adult measurements (mm): SVL 39.8–48.0 (43.1); TL 19.5–22.8 (20.7); FL 9.8–11.8 (10.6); HW 14.5–17.5 (15.9); HL 10.1–11.7 (11.0); ED 3.7–4.7 (4.2); EN 3.2–3.8 (3.5); IN 3.2–4.2 (3.6); 3DW 2.3–3.1 (2.7); 3FL 7.0–8.1 (7.4); 4TL 9.3–11.9 (10.5). Adult proportions: TL/SVL 0.45–0.52 (0.48); FL/SVL 0.23–0.27 (0.25); FL/TL 0.48–0.58 (0.51); HW/ SVL 0.34–0.41 (0.37); HL/SVL 0.24–0.27 (0.25); HW/HL 1.34–1.55 (1.45); ED/SVL 0.08–0.11 (0.10); EN/IN 0.86–1.11 (0.99); EN/ED 0.71–0.93 (0.84); 3DW/SVL 0.054–0.068 (0.062); 3FL/SVL 0.16–0.19 (0.17); 4TL/SVL 0.22–0.27 (0.24). Comparison of sexes: Females are on average larger than males (SVL 43.7 vs. 42.5) but the ranges overlap substantially (females: 39.8–48.0 mm, males 40.5–44.8 mm). No differences in proportions were detected between the sexes. Juvenile measurements (mm): SVL 24.2; TL 12.2; FL 5.5; HW 9.1; HL 7.0; ED 2.5; EN 2.2; IN 2.0; 3DW 1.5; 3FL 4.2; 4TL 5.8. Juvenile proportions: TL/SVL 0.50; FL/SVL 0.23; FL/TL 0.45; HW/ SVL 0.38; HL/SVL 0.29; HW/HL 1.31; ED/SVL 0.10; EN/IN 1.14; EN/ED 0.88; 3DW/SVL 0.060; 3FL/SVL 0.18; 4TL/SVL 0.24. Compared to adults, the juvenile has a proportionally longer head and shorter forearms. Head: Narrower than body, triangular in dorsal view; snout truncated at the nares, noticeably projecting in profile; canthus rostralis angular, loreal region steep; nares much closer to tip of snout than to eye, nares anterolateral on tip of snout; eyes large; eye diameter greater than eye to naris distance; internarial distance about equal to distance from eye to naris; tympanum large (approximately half to greater than half diameter of eye) but indistinct beneath overlying skin, bordered dorsally by supra-tympanic fold. Body: Rotund. Limbs: Hindlimbs and forearms relatively long; fingers and toes unwebbed; relative finger length 3>4>2>1; fingers 2, 3 and 4 long and slender with very large and obviously truncated discs, first finger short with disc expanded but small and rounded; low, ovoid inner and larger, rounded outer palmar tubercles; subarticular tubercles low, moderately prominent; relative length of toes 4>3>5>2>1, toe 4 very long and slender; large, truncated discs on toes 2, 3 and 4, discs smaller and more rounded on toes 1 and 5; low, rounded inner metatarsal tubercle, no outer metatarsal tubercle; subarticular tubercles low and rounded, moderately prominent; discs on longest fingers larger than discs on longest toes. Skin: Ventral surface smooth; dorsal surface of head and limbs covered in very fine tubercles, back covered with scattered low tubercles; distinct supra-tympanic fold. Colour pattern in preservative: All dorsal surfaces uniformly dark brown. Tympanum tawny brown with darker centre. White patches at the base of the finger and toe discs. Dark brown dorsal colouration merges to lighter brown on flanks. Ventral surface of head and body light grey-brown. Ventral surfaces of limbs brown; discs and tubercles grey. The orange seen in life in the groin, posterior thigh and on the calf appears as white patches in preserved specimens.
Measurements of live individuals. Two adults were measured in the field: female, SVL 44.3 mm, WT 8.1 g; male 42.4 mm, 6.1 g.
Colour pattern in life ( Fig. 3 View FIGURE 3 ). Adults. No sexual dimorphism in colour pattern evident. Dorsal colour variable. Some individuals emerging from rock crevices were dark brown ( Fig. 3 View FIGURE 3 B) and most individuals changed to this colouration in bags following capture. Individuals active on the surface of the rocks or in associated vegetation were even light brown, uneven grey-brown ( Fig. 3 View FIGURE 3 C) or a fairly uniform pale putty colour ( Fig. 3 View FIGURE 3 A). Diffuse, pale lumbar ocelli are present on some individuals. There is a dark canthal band from the nare to the eye, through the iris, and along the supra-tympanic fold. The upper iris is heavily speckled with gold or copper; speckling is present but less intense in the lower iris. Most individuals have a paler brown or yellowish forehead triangle and similar colouration on the eyelids. The darker dorsal colouration merges with the paler ventral colouration on the flanks. The ventral surfaces are uniformly white on some individuals, while on others they are pale with heavy grey mottling on the chin, throat, chest and forearms ( Fig. 3 View FIGURE 3 D). The palms are dark. Reddish orange markings are present in the hidden parts of the hindlimbs (in the groin and on the posterior thigh and inner calf) ( Figs 3 View FIGURE 3 C, 3D). The brightness and extent of these markings varies between individuals, from very bright and discrete to more diffuse. Juveniles. Greyish brown to yellowish colour with darker mottling on the back, arms and legs. There is a yellowish brown forehead triangle, a dark canthal streak and dark markings on the loreal region, above the tympanum and on the anterior flank. Yellow lumbar ocelli are evident. The flanks and ventral surfaces are mottled grey, and the groin, posterior thigh and calf are orange.
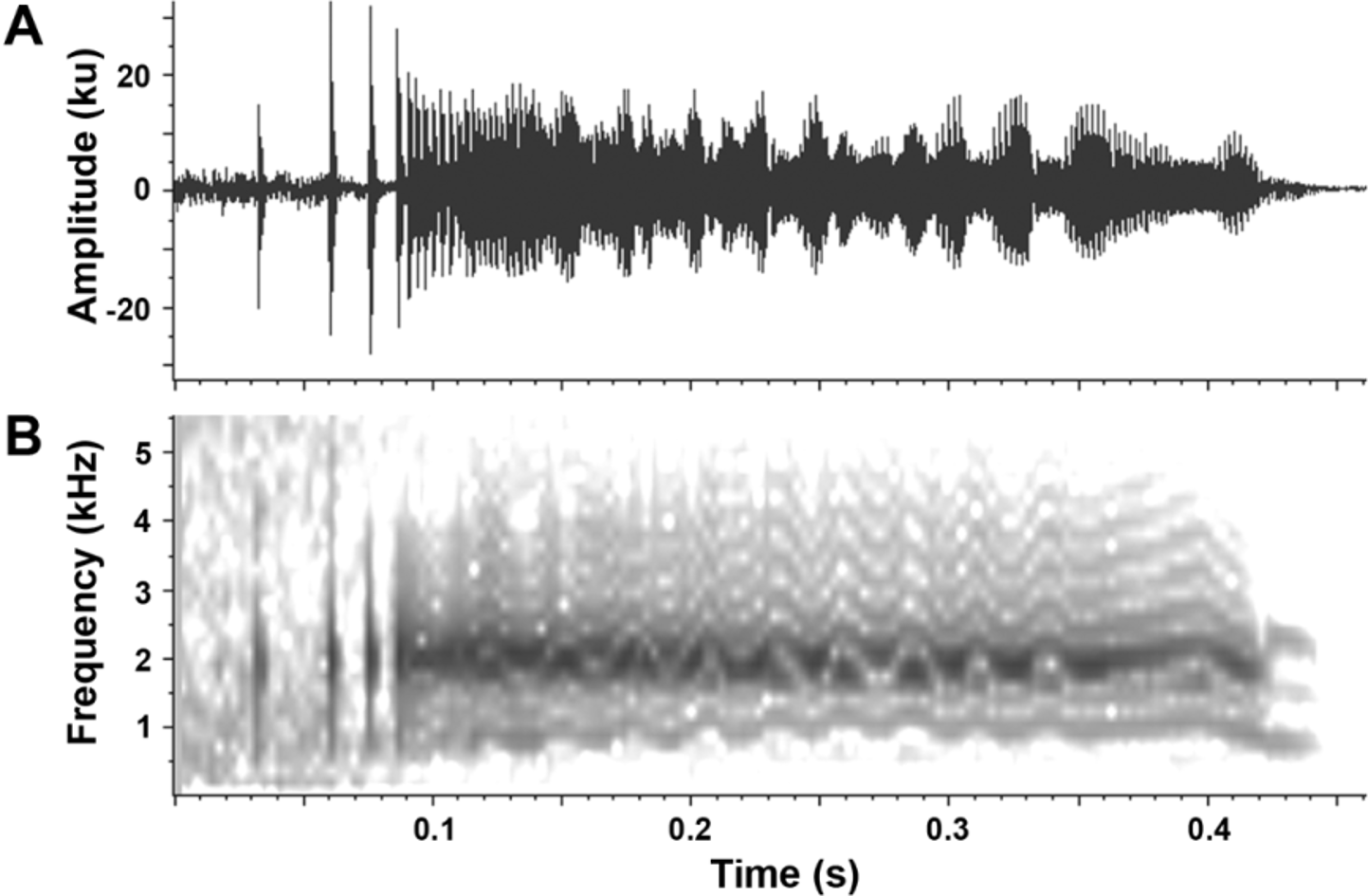
Call. Calls were only obtained for a single individual, which was not collected. No obvious differences were heard between the calls of this individual and those calling around it. The mating call of C. kulakula sp. nov. is a short, wavering ‘bleat’ of the following mean characteristics: dominant frequency 1.97 kHz (1.95–1.99), duration 0.38 s (0.35–0.41), pulses per call 155 (140–167), and pulse rate 411 pulses/s (381–448). Calls are uttered at an interval of approximately 8 seconds. Air temperature at the time of recording was approximately 28 ºC. Figure 4 View FIGURE 4. A displays a single representative call.
The call is distinct from that of other Australian Cophixalus (although the calls of C. pakayakulangun sp. nov. and C. zweifeli are not known). It is obviously different to the tapping calls of most Australian Cophixalus (including C. saxatilis ) and is most similar to the ‘bleat’ of C. ornatus . The call of C. ornatus is a loud, ‘clean’, highpitched ‘beep’, whereas that of C. kulakula sp. nov. is relatively soft, deeper in pitch and has a wavering, ‘scratchy’ quality to it. The call is of higher dominant frequency than would be expected for body size, compared to the relationship seen across other Cophixalus and Australian frogs in general ( Hoskin et al. 2009).
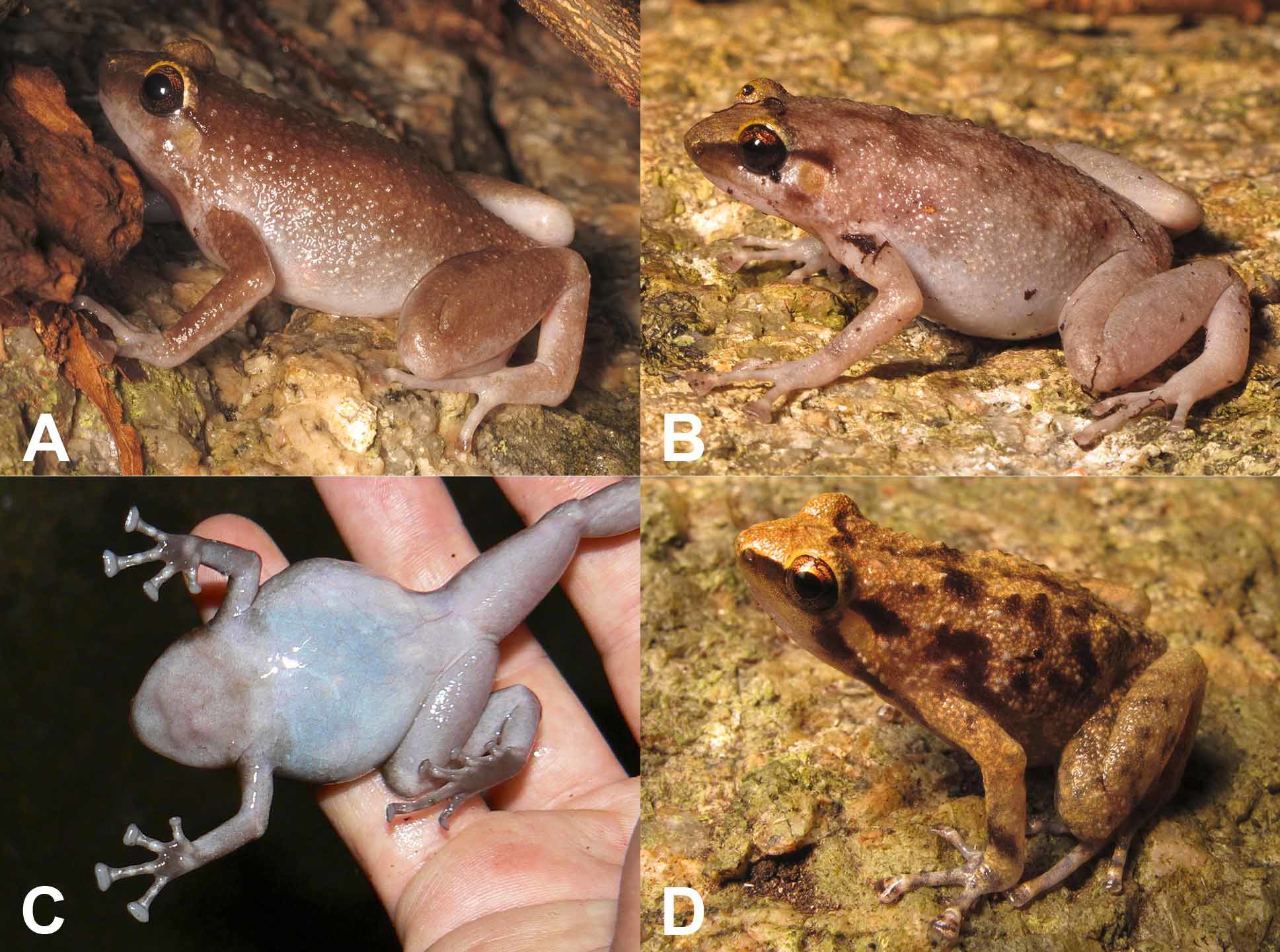
Comparison. Cophixalus kulakula sp. nov. does not co-occur with any other Cophixalus . It could only be confused with the other three boulder-adapted Cophixalus : C. pakayakulangun sp. nov. (Stanley Hill region, 30 km to the north), C. zweifeli (Cape Melville, 210 km south-east) and C. saxatilis (Black Mountain, 390 km south-east). These four species can be distinguished as follows. Cophixalus saxatilis differs from the other species in that females are bright yellow ( Fig. 1 View FIGURE 1 A) and males are heavily mottled and relatively small (29–35 mm) ( Fig. 1 View FIGURE 1 B). Cophixalus zweifeli ( Figs 1 View FIGURE 1 C, 1D) and C. kulakula sp. nov. have red/orange in the groin and on the posterior thigh, whereas this is at most a faint wash on adult C. pakayakulangun sp. nov.. Cophixalus zweifeli differs from C. kulakula sp. nov. in that it also has red/orange in the axilla (absent in C. kulakula sp. nov.) and dark blotching on the anterior half of the flanks (i.e. above the axilla) ( Figs 1 View FIGURE 1 C, 1D), whereas the flanks of C. kulakula sp. nov. are generally unmarked ( Fig. 3 View FIGURE 3 ). Additional differences between C. pakayakulangun sp. nov. and C. kulakula sp. nov. are that C. pakayakulangun sp. nov. females are larger (SVL 49–53 mm vs. 40–48 mm), there is generally an obvious gold tinge to the eyelids and forehead of C. pakayakulangun sp. nov. ( Figs 6 View FIGURE 6 A, 5B) whereas this is faint or absent on C. kulakula sp. nov. ( Figs 3 View FIGURE 3 A–C), and the juveniles of C. pakayakulangun sp. nov. are blotched with dark markings ( Fig. 6 View FIGURE 6 D) whereas those of C. kulakula sp. nov. are mottled with darker markings but not obviously blotched.
Genetics. There is substantial sequence divergence (for 944 bp 12S and 16S rRNA) between C. kulakula sp. nov. and all other Australian Cophixalus (average = 12.1%, range = 8.3–14.7%) (Hoskin et al. unpublished). Phylogenetic analyses suggest that Cophixalus kulakula sp. nov. is most closely related to C. pakayakulangun sp. nov., and these two species are allied to C. ornatus (Hoskin et al. unpublished). Sequence divergence between C. kulakula sp. nov. and C. pakayakulangun sp. nov. is high (8.3%) compared to that between some sister-species of north Queensland Cophixalus (e.g., 4.2% between C. concinnus Tyler, 1979 and C. monticola Richards, Dennis, Trenerry & Werren, 1994 ; 5.1% between C. aenigma Hoskin, 2004 and C. exiguus Zweifel & Parker, 1969 ). A 16S sequence for C. kulakula sp. nov. was deposited in GenBank (accession number JN208371 View Materials ).
Distribution. Cophixalus kulakula sp. nov. is known from three sites in the vicinity of Mt Tozer in north-east Queensland ( Fig. 2 View FIGURE 2 ). One of the sites is on the eastern side of Mt Tozer, another is on the north-western slopes of the Mt Tozer uplands, and the third is a hill to the north of ‘Tozer’s Gap’, approximately 5 km NW of Mt Tozer. There are other patches of boulder habitat in the Mt Tozer area and in the adjacent rocky hills to the north known as “The Paps”, and C. kulakula sp. nov. probably inhabits many of these. A fourth known site, provisionally assigned to C. kulakula sp. nov. (see Discussion), is Tor Hill, approximately 25 km SW of Mt Tozer.
Habitat and habits. Cophixalus kulakula sp. nov. inhabits deeply piled granite boulder habitats and its morphology is similar to that of the other three boulder-adapted Australian Cophixalus . These species are all relatively large and have long fingers and greatly enlarged finger discs. As for the other species, C. kulakula sp. nov. is restricted to boulder habitats. These are either boulder fields or deeply piled rock in rainforest gullies ( Fig. 5 View FIGURE 5 ). At most sites the boulders are festooned with vegetation such as ferns, vines ( Scindapsus altissimus and others) and umbrella trees ( Schefflera ). The frogs emerge from deep crevices on dusk to forage at night on the surface of rocks and on associated vegetation (particularly Schefflera ) ( Fig. 5 View FIGURE 5 ). Some individuals were observed in the crowns of umbrella trees up to 2.4 metres above the rocks below. No other frogs were observed in boulder habitats at these sites. Analysis of C. kulakula sp. nov. scats (removed from holding bags in the field) revealed that they feed primarily on ants; as has been found for other Australian Cophixalus ( Hoskin 2004; Williams et al. 2006). An adult C. kulakula sp. nov. was observed feeding on Oecophylla smaragdina ants by picking individual ants from an ant trail. Other invertebrate groups represented in the scats were Coleoptera and Blattodea.
Cophixalus kulakula View in CoL sp. nov. were abundant at sites in February 2009, April 2010 and December 2010, with males and females being observed in approximately equal numbers. Males were calling in December but not in February and April, despite wet weather during all visits. This suggests that breeding occurs earlier in the wet season. Juveniles (approx. 25 mm) were observed at one site in April. Males were observed calling from the tops and sides of boulders. Males were calling in small groups, with males spaced several metres from each other, and such groups were widely separated despite apparently suitable boulder habitat in between. A calling male was observed to lead a female from his calling site in piled boulders to a crevice below an isolated small boulder approximately 40 metres away on the rainforest floor. The male uttered a call shorter than the mating call (described above) while leading the female. This behaviour is similar to that reported for C. ornatus View in CoL , in which males lead females some distance from a calling site to a nest site ( Zweifel 1985; Hoskin 2004; Felton et al. 2006). As for other Australian microhylids ( Hoskin 2004), C. kulakula View in CoL sp. nov. is almost certainly a terrestrial breeder. A female preserved in January contained 47 unpigmented ova of an average diameter of 2.8 mm (2.7–2.9). These eggs were all of similar size and appeared to represent one clutch. If this were the case, then this represents a substantially larger clutch size than recorded for other Australian microhylids (6– 22 eggs, average 12), including that recorded for another large boulder-dwelling species, C. saxatilis View in CoL (13 eggs) ( Hoskin 2004).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |