Reticulipeurus (Forcipurellus), Gustafsson & Zou, 2023
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5284.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:DED5B7B1-123A-483D-93B0-803D0D1D05EF |
|
DOI |
https://doi.org/10.5281/zenodo.7929643 |
|
persistent identifier |
https://treatment.plazi.org/id/039687AE-FFAC-FFDB-FF4D-F8D5FB0DFF5F |
|
treatment provided by |
Plazi |
|
scientific name |
Reticulipeurus (Forcipurellus) |
| status |
subgen. nov. |
Forcipurellus new subgenus
Type species: Lipeurus formosanus Uchida, 1917 View in CoL .
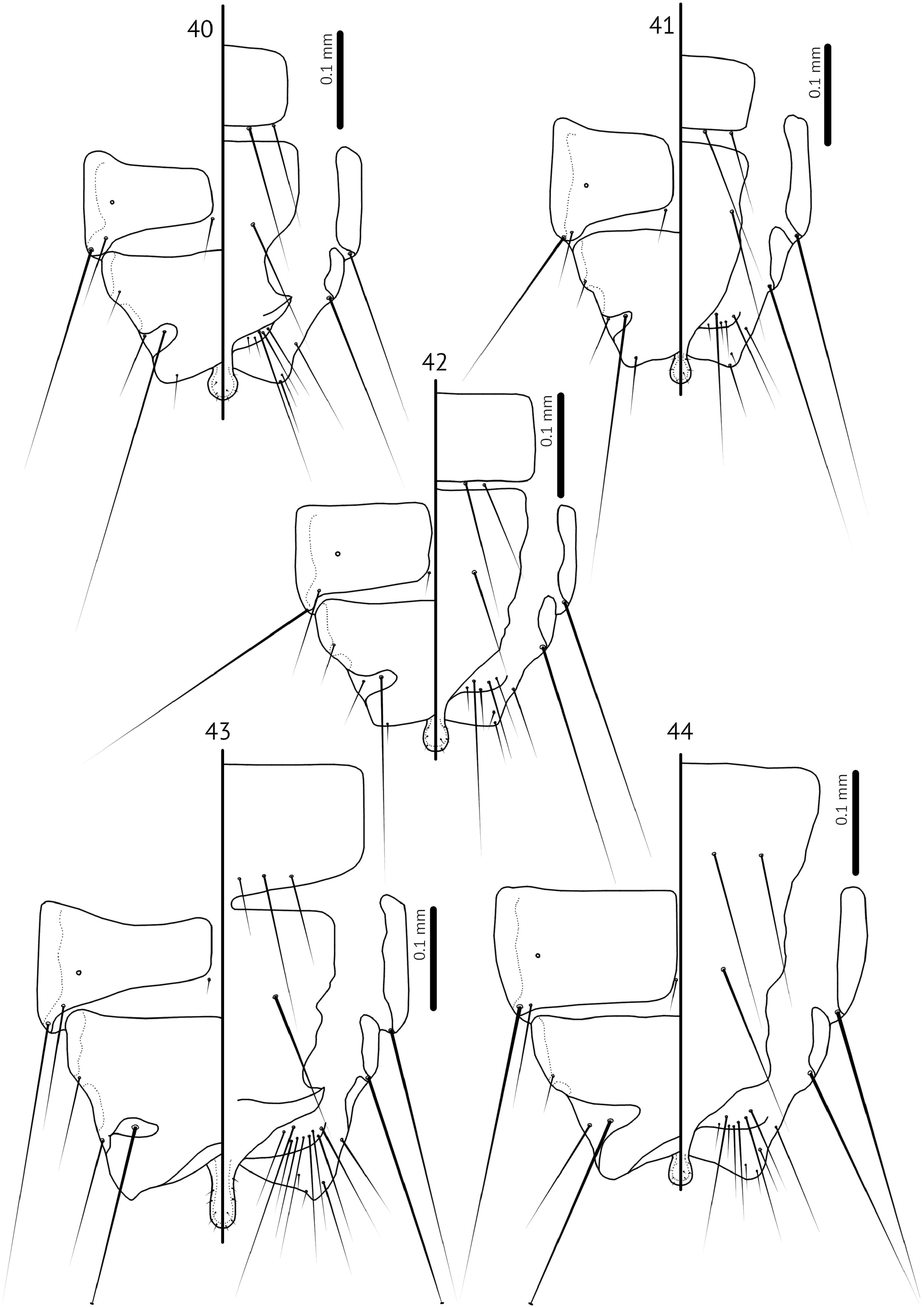
Diagnosis. Members of the subgenus Forcipurellus can be separated from members of the nominate subgenus by the following combination of characters [see Gustafsson et al. (2020b) for illustrations of the nominate subgenus]: male stylus subterminal in R. ( Reticulipeurus ), but terminal in R. ( Forcipurellus ) (e.g. Fig. 43 View FIGURES 40–44 ); distal end of female abdomen with prominent, typically medianly curved, “claspers” in R. ( Forcipurellus ) (e.g. Figs 8 View FIGURES 3–8 , 16 View FIGURES 11–16 , 23 View FIGURES 19–23 ), but without such structures in R. ( Reticulipeurus ); female vulval margin in R. ( Forcipurellus ) with distinct lateral lobes and more or less convex median section (e.g. Figs 8 View FIGURES 3–8 , 16 View FIGURES 11–16 , 23 View FIGURES 19–23 ), whereas in the nominate subgenus the vulval margin is either gently concave without distinct lateral lobes and median convex section, or if lateral sections are lobe-like, then median section is not convex. In general, reticulation patterns in R. ( Forcipurellus ) are not as extensive as in some species of the nominate subgenus, but this varies among species in R. ( Reticulipeurus ).
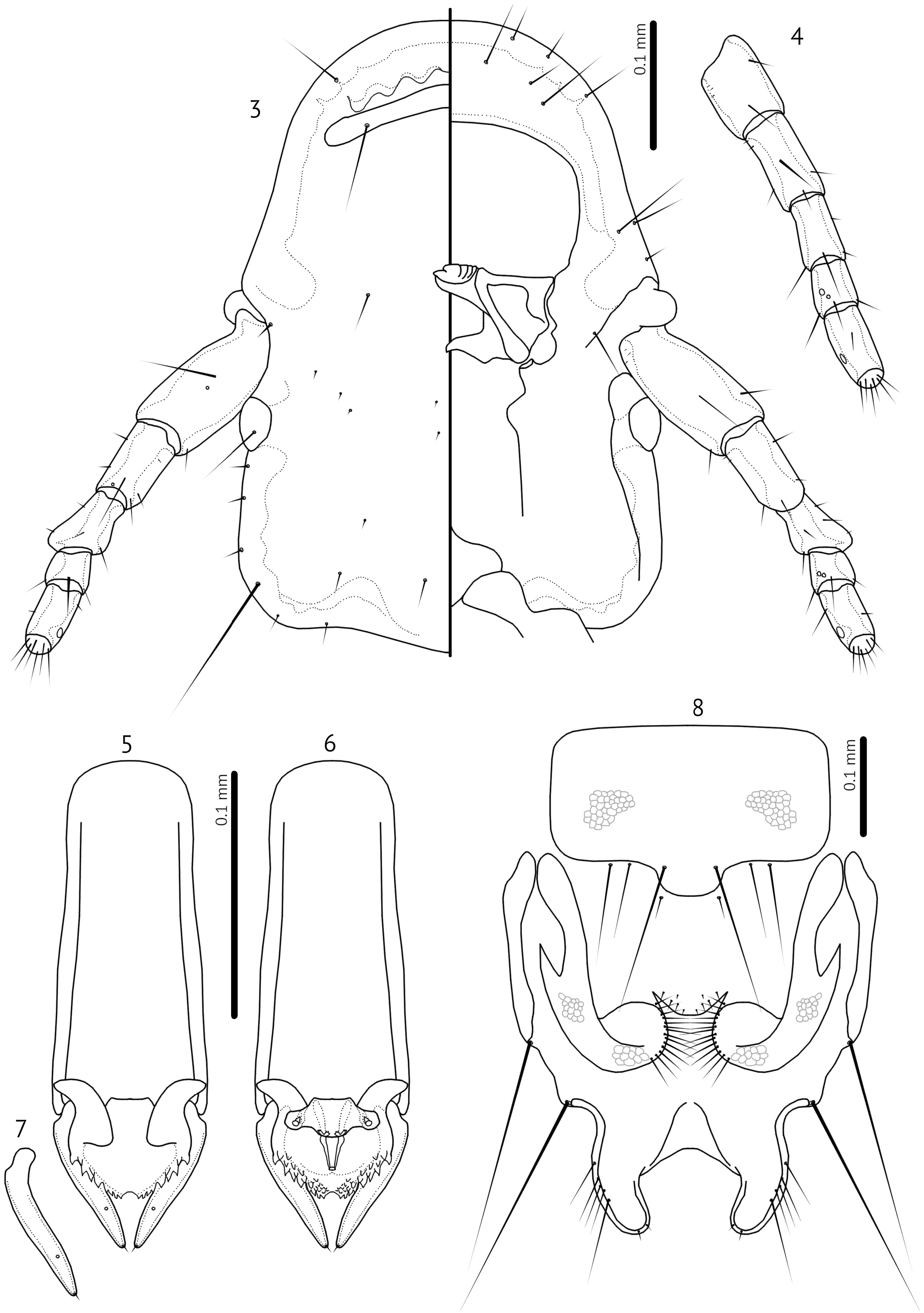
Description. Both sexes. Generally small, slender species with oblong heads ( Fig. 3 View FIGURES 3–8 ). Frons rounded, marginal carina uninterrupted. Internal sinuous thickenings present near frons. Dorsal preantennal suture present, reaching anterior dorsal seta. Head chaetotaxy as in nominate subgenus, except that head sensilla s1–4, s6–8 all present, and s2 is farther anterior to s1 than in nominate subgenus; mandibular seta as mesosetae. Coni small. Antennae sexually dimorphic: male scape, pedicel and flagellomere I enlarged, not in female, but degree of male modification variable among species.
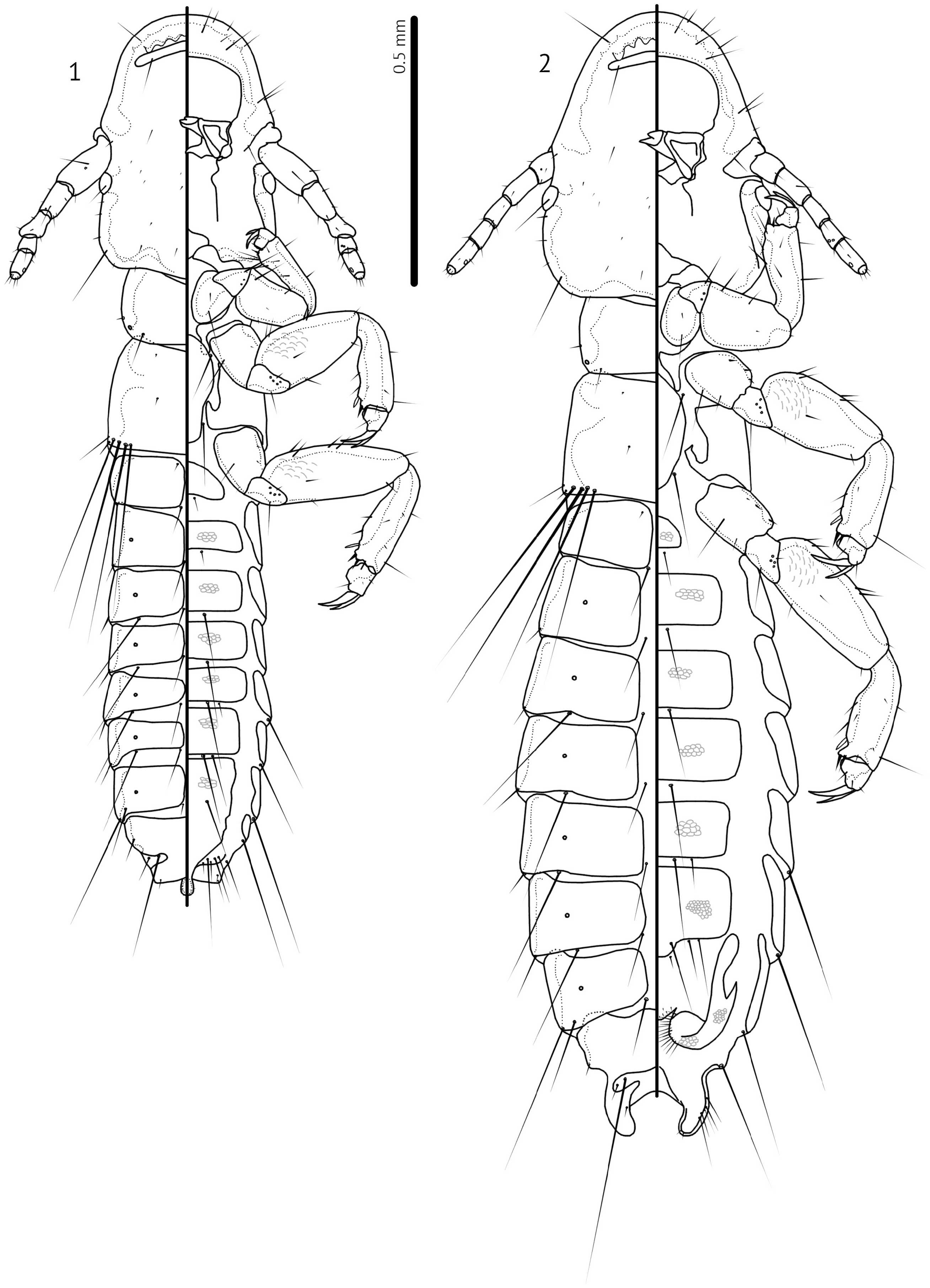
Pronotum with 1 pronotal marginal-lateral seta (pmls) and 1 pronotal post-spiracular seta (ppss) on each side; pronotal dorsal anterior seta (pdas) may be present as in nominate subgenus, but if present too small to see in examined specimens. Pteronotum with 1 anterior and 1 posterior submarginal meso-metanotal seta (asmns and psmns, respectively), 1 pterothoracic trichoid seta (ptrs) and 1 pterothoracic thorn-like seta (pths) on each side. Marginal mesometathoracic setae (mms) in single group of four setae on each side, widely separated medianly ( Figs 1–2 View FIGURES 1–2 ). Leg chaetotaxy largely as in nominate subgenus, but setae fII-d1, fII-a3, fIII-d1, and fIII-a3 more marginal, and tbII-p3 and tbIII-p3 hyaline; tI-v3 not clearly visible in examined specimens, but may be present.
Abdomen slender, with tergopleurites II–VIII medianly divided and tergopleurites IX–XI fused ( Figs 1–2 View FIGURES 1–2 ). Central sternal plates present on segments II–VI or II–VII (males of some species), but poorly sclerotised. Abdominal chaetotaxy sparse, identical in all species.
Male. Antennal scape swollen, about twice as long as female and much wider in some species, with basal modifications (e.g. Fig. 11 View FIGURES 11–16 ); pedicel may be modified in shape compared to that of female (e.g. Fig. 12 View FIGURES 11–16 ), but roughly similar in size; flagellomere I may be swollen or extended distally (e.g. Fig. 3 View FIGURES 3–8 ), but not in all species ( Fig. 19 View FIGURES 19–23 ); flagellomere I may also be elongated and slightly curved (Fig, 11). Distal end of abdomen concave (e.g. Figs 40–44 View FIGURES 40–44 ). Subgenital plate with terminal stylus that reaches beyond distal margin of abdomen (e.g. Figs 40–44 View FIGURES 40–44 ); fusion of subgenital plate and sternal plate VII not clearly visible, and may be variable between species as illustrated here (e.g. Figs 40–44 View FIGURES 40–44 ). Genitalia: Basal apodeme slender; mesosome with hooked antero-lateral extensions overlapping with basal apodeme; distal margin of mesosome rugose; ventral sclerite proportionately larger than is normal in nominate subgenus, but structurally similar, with lateral extensions bearing 1–2 gonoporal posterior mesosomal setae (gpmes) visible at sensilla on each side; gonopore elongate, not fused distally; parameres slender, with blunt or somewhat elongated heads with parameral seta 1 (pst1) as sensillus and parameral seta 2 (pst2) as microseta near apical end of paramere (e.g. Figs 5–7 View FIGURES 3–8 , 13–15 View FIGURES 11–16 ).
Female. Abdominal segments IX–XI fused, with distal end forming medianly curved “claspers” (e.g. Figs 8 View FIGURES 3–8 , 16 View FIGURES 11–16 , 23 View FIGURES 19–23 ). Anal setae divided into 1 dorsal and 2 ventro-marginal on each side. Sternal plate VII generally rectangular but may bulge distally in median section ( Fig. 2 View FIGURES 1–2 ); however, sternal and subgenital plates are generally poorly sclerotised and outlines are illustrated approximately. Subgenital plate with medianly divided cross-pieces on each side, following the vulval margin laterally. Vulval margin medianly convex (e.g. Figs 8 View FIGURES 3–8 , 16 View FIGURES 11–16 , 23 View FIGURES 19–23 ), with small number of slender vulval submarginal setae (vss); laterally; the vulval margin forms a distinct rounded lobe on each side, with numerous slender vulval marginal setae (vms) on each side. One, rarely two, short setae on each side situated between subgenital plate and vulval margin may represent the vulval oblique setae (vos). Vulval margin laterally with sclerotised cross-piece, which is not fused medianly, and may be reticulated partially. Subvulval plates not visible, but may be poorly sclerotised.
Host distribution. All known species of Forcipurellus parasitise species of the genus Arborophila Hodgson, 1837 ( Galliformes : Phasianidae ).
Geographical range. Most of the known species of Forcipurellus span the range of the host genus, from Taiwan and Sumatra to Sikkim in India.
Etymology. The name Forcipurellus is constructed by “ forcipatus ” Latin for “pincer”, “ ourá ” Greek from “tail”, and ellus ” Latin for “small”. The first two words refer to the prominent claspers of the distal female abdomen, which separate all members of this subgenus from other species of Reticulipeurus , with the addition of “ ellus ” referring to the size of Forcipurellus species, generally smaller than those of the nominate subgenus.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.