Octodontomys gliroides (P. Gervais and (P. Gervais and d'Orbigny, 1844)
|
publication ID |
https://doi.org/ 10.1093/mspecies/sey010 |
|
publication LSID |
lsid:zoobank.org:pub:B8876E05-78A8-45FA-A34A-9FFB56741A20 |
|
DOI |
https://doi.org/10.5281/zenodo.4592306 |
|
persistent identifier |
https://treatment.plazi.org/id/03970124-E858-FFE6-FF5B-B08A2EBC056C |
|
treatment provided by |
Felipe |
|
scientific name |
Octodontomys gliroides (P. Gervais and |
| status |
|
Octodontomys gliroides (P. Gervais and View in CoL d’Orbigny, 1844)
Mountain Degu
Octodon gliroides P. Gervais and d’Orbigny, 1844:22 . Type locality “des Andes boliviennes, à Lapaz;” La Paz, Bolivia.
Neoctodon simonsi Thomas, 1902:115 . Type locality “Mountainous region south and south- east of the Titicaca- Poopo basin. Potosí, 4400 metres,” Bolivia.
Octodontomys gliroides: Thomas, 1913:143 View in CoL . First use of current name combination.
CONTEXT AND CONTENT. Context as for genus. Octodontomys gliroides View in CoL is monotypic ( Verzi et al. 2015a).
DIAGNOSIS
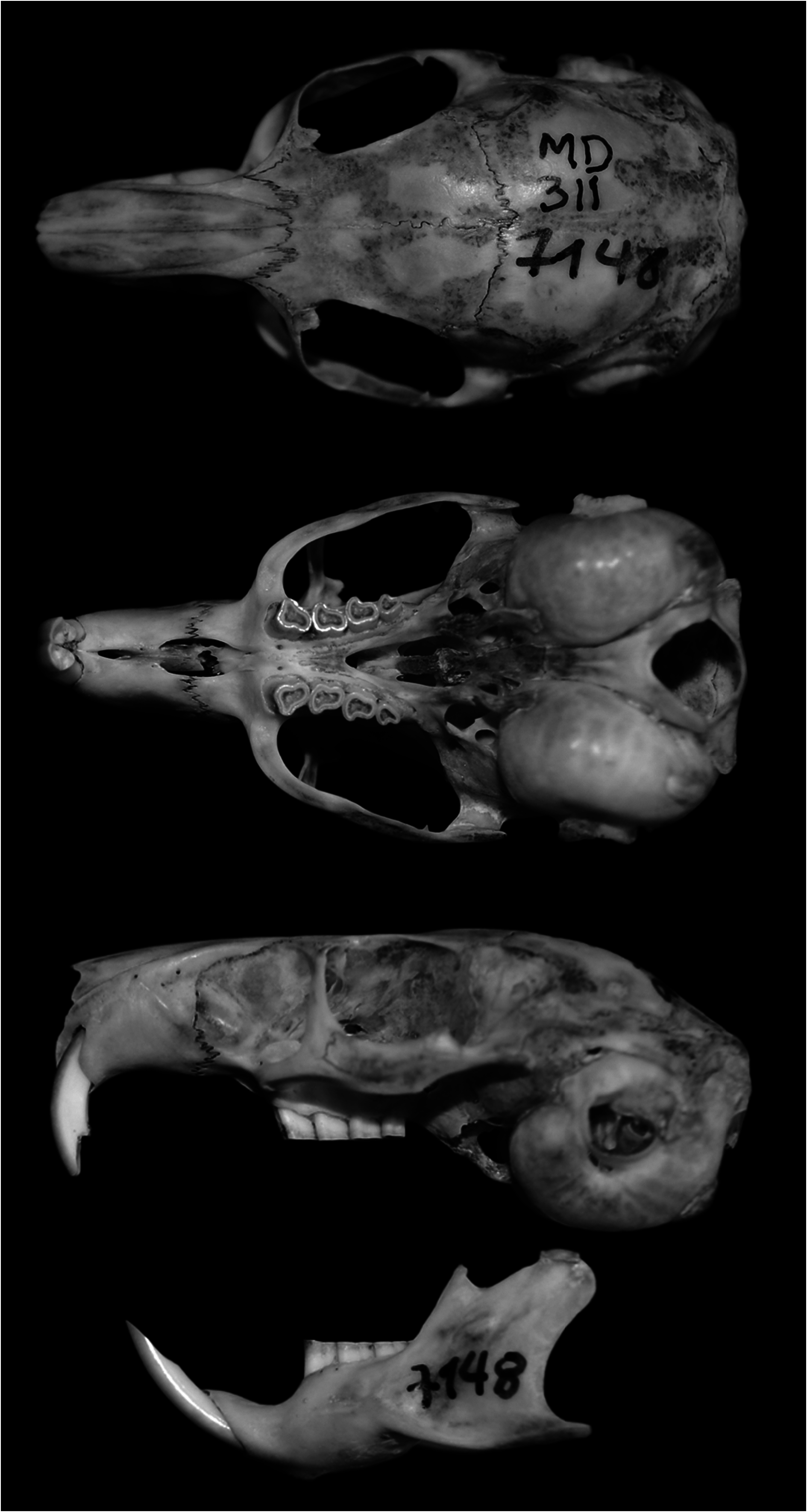
Octodontomys gliroides ( Fig. 1 View Fig ) resembles other members of the family Octodontidae , being most similar to the mountain viscacha rat Octomys mimax and species of the genus Tympanoctomys ; however, O. gliroides has upper and lower molariforms with an asymmetrical occlusal pattern ( Octomys and Tympanoctomys have a figure-8 occlusal pattern—Reig and Quintana 1991; Hutterer 1994; Kramarz 2005). Other distinctive features of the teeth and skull of O. gliroides ( Fig. 2 View Fig ) include: the alveolar portion of the 1st molariform tooth (DP4) is slightly inclined anteriorly, medial to the incisor; as a result, an anterior extension of the maxillaries into the posterior margins of the incisive foramina, where DP4 is inserted, is scarcely developed to nearly absent ( Verzi 2001; Verzi et al. 2015a). The molariform teeth have folds (flexi or flexids) that are shallow or absent; the maxillary cheek teeth have the anterior lobe protruding as in Octodon , but without a penetrating lingual fold (hypoflexus— Verzi and Carrín Iglesias 1999; Verzi 2001; Verzi et al. 2015a). The auditory bullae are of medium size (14.6 ± 0.7 mm) and slightly inflated compared with other desert-adapted octodontids such as Octomys (15.8 ± 0.3 mm) and Tympanoctomys (17.2 ± 0.9 mm—Mares et al. 2000).
GENERAL CHARACTERS
Octodontomys gliroides is a medium- to large-sized octodontid, with a body mass ranging from 100–200 g; total length, 200–380 mm; length of tail, 100–190 mm; length of hind foot, 33–40 mm; length of ear, 24–35 mm (Díaz 1999; Mares et al. 2000; Díaz and Barquez 2002; Verzi et al. 2015a). It is a semisubterranean genus of the family Octodontidae and, like Octodon and Tympanoctomys , has large eyes, ears, and hind feet (Lacey and Ebensperger 2007). The pelage is relatively long and silky; dorsal coloration is grayish drab streaked with black; tail has a reddish buffy brush; the venter has white hairs with gray bases, except on the chin and throat where they are pure white, which contrasts sharply with the dorsum ( Thomas 1902; Ipinza et al. 1971; Mares et al. 2000; Verzi et al. 2015a). The ears are large, finely covered by short grayish hairs, with white tufts on the anterior ends ( Ipinza et al. 1971; Díaz and Barquez 2002). Feet are hairy with the claw covered by hair, the plantar surfaces bear fine granulations ( Thomas 1902; Contreras et al. 1987; Díaz and Barquez 2002), and plantar pads are prominent ( Ipinza et al. 1971). The tail is nearly 80% of the head and body length; it is bicolored, with an elongated, brown or ochraceous brush extending from the tip. Juveniles are darker with a grayer venter and have an essentially unicolored tail that terminates in a short, black or dark drab brush (Díaz and Barquez 2002; Verzi et al. 2015a).
The rostrum is relatively long and narrow; postorbital processes are absent or reduced; interorbital region is relatively broad and divergent with breadth slightly less than the breadth of the rostrum (9.23 mm versus 7.41 mm); lacrimal is large and triangular; palate is short, and the mesopterygoid fossa has an inverted “V” shape, not extending beyond M1; pterygoids leaning on the bullae (Díaz 1999; Mares et al. 2000). Incisive foramina are large with the posterior most ends behind the premaxillary-maxillary suture; the lateral edges of the posterior bor- der of the incisive foramina are not raised ( Mares et al. 2000). The lateral flange of the canal for the infraorbital nerve is well developed and has the dorsal end free, not joined to the maxillary (Glanz and Anderson 1990; Verzi et al. 2015a). Masseteric crest and the tubercle for the insertion of the M. masseter medialis pars infraorbitalis are discontinuous (Verzi et al. 1999, 2001; Kramarz 2005). The zygomatic arches are little expanded, with a low and slightly deep jugal fossa for the posterior masseter muscle; shape of suture of jugal and zygomatic process of squamosal squared or rounded ( Mares et al. 2000; Verzi et al. 2015a). The lateral supraoccipital processes show greatest development, with the long process extending to the squamosal process and then turning conspicuously downward (Glanz and Anderson 1990). Woods (1984:424, 428) mentioned a “slight lateral supraoccipital process” in both Ctenomyidae and Octodontidae , but Glanz and Anderson (1990) considered this description slightly misleading because the supraoccipital process is well developed in O. gliroides . The paraoccipital processes are small, thick, and incompletely joined to the bullae, with each root directed medially due to the development of the mastoid bullae ( Mares et al. 2000; Verzi et al. 2015a).
The mandible is slender, low, and moderately hystricognathous; the coronoid process is small; the condylar process is broad and slightly behind the angular process, the latter being short, slender, and slightly flattened; the lunar notch is shallow (Díaz 1999; Mares et al. 2000; Verzi et al. 2015a); the masseteric crest is not prominent ( Verzi 2002).
Mean cranial measurements (mm, ± SD, sample size in parentheses—Mares et al. 2000, specimens from Argentina and Bolivia) were: greatest length of skull, 45.3 ± 1.3 (25); basal length, 39.6 ± 1.3 (22); zygomatic breadth, 23.3 ± 0.7 (26); mastoid breadth, 22.1 ± 0.7 (21); least interorbital breadth, 9.4 ± 0.4 (29); length of nasals, 16.6 ± 0.9 (26); breadth of rostrum, 8.2 ± 0.3 (26); length of diastema, 11.5 ± 0.5 (28); length of maxillary toothrow, 8.1 ± 0.6 (28); length of bulla, 14.6 ± 0.7 (23); width of bulla, 11.3 ± 0.3 (23); width of zygomatic plate, 1.9 ± 0.2 (26); length of mandibular toothrow, 8.5 ± 0.6 (27); length of mandible, 27.5 ± 0.9 (28). Mean cranial measurements (mm, ± SD, sample size in parentheses—Díaz 1999, specimens from Argentina) were: condyle incisive length, 42.2 ± 1.2 (11); occipitonasal length, 43.8 ± 1.5 (10); braincase breadth, 17.9 ± 0.5 (11); zygomatic breadth, 23.0 ± 0.8 (10); mastoid breadth, 14.2 ± 2.1 (9); least interorbital breadth, 9.3 ± 0.4 (11); length of rostrum, 17.9 ± 0.7 (9); breadth of rostrum, 7.8 ± 0.5 (10); length of nasals, 15.9 ± 0.9 (4); length of diastema, 11.5 ± 0.4 (11); length of maxillary toothrow, 8.7 ± 0.5 (11); greatest alveolar length of M1, 2.7 ± 0.2 (10); alveolar width of M1, 2.08 ± 0.11 (6; please note this was original reported incorrectly as 4 ± 5.7 (10)); greatest length of incisive foramina, 4.07 ± 0.7 (10); palate length, 16.8 ± 0.7 (11); maxima distance between the external border of M3, 7.5 ± 0.5 (11); length of bulla, 14.0 ± 0.9 (10); length of mandibular toothrow, 9.2 ± 0.5 (11); length of mandible, 25.8 ± 1.2 (11). These 2 cited publications differ in mastoid breadth; it is possible that Mares et al. (2000) included part of the bulla in their measurement.
The upper incisors are orthodont or slightly proodont, orange, narrow, and short; the lower incisors are orange, high, narrow, with a suboval section, straight lingual enamel edge, and curved labial edge; on the labial side, the enamel extends beyond one-half of the tooth face (Verzi et al. 1999; Verzi 2002). Like all octodontids, O. gliroides is characterized by ever-growing (hypsodont) molar teeth. In the upper molariforms, the folds (flexi) are absent in the adult and the posterior lobe is not extended labially. The molariforms are asymmetrical with no reentrant folds ( Hutterer 1994), except in juveniles, which have flexids and fossettids ( Verzi 1994; Verzi et al. 2016). Lower molariform teeth have an asymmetrical occlusal pattern, nearly figure-8 shaped, with slightly oblique and rounded lobes and shallow traces of folds; deciduous dp4 with mesoflexid reduced or absent and hypoflexid barely visible ( Verzi 1994; Verzi et al. 2015a); the mesoflexid of m1 and m2 remains open in advanced stages of wear ( Kramarz 2005); with mesoflexid less evident than hypoflexid ( Verzi 1994); last upper and lower molars (M3/m3) reduced in size ( Verzi 2002; Verzi et al. 2015a). O. gliroides has oblique, posterolabial-anterolingual, unilateral chewing (anterolingual jaw displacement and alternate occlusion); according to phylogenetic analyses, this is primitive for octodontids ( Verzi 2001; Olivares et al. 2004). The masticatory direction (in molariform teeth, defined as asymmetry in dentine wear relative to the sagittal plane) is 58° in Octodontomys (Vassallo and Verzi 2001) . Hutterer (1994) considered the occlusal pattern of Octodontomys to be plesiomorphic, because it matches with the molars of the Oligocene Platypittamys , a putative octodontid. The similarities in the cranial morphology of O. gliroides and the basal octodontoid rodent Prospaniomys make O. gliroides an optimal species for reconstructing masticatory muscle origins and insertions of this extinct species (Álvarez and Arnal 2015).
The data matrix of Olivares et al. (2012) and Verzi et al. (2016) should be consulted for additional cranial and dental characters for O. gliroides . Álvarez et al. (2011) and Álvarez et al. (2015) examined 2- and 3-dimensional craniomandibular geometric morphometric characters in an evolutionary context.
DISTRIBUTION
Octodontomys gliroides has a disjunct distribution ( Fig. 3 View Fig ) in the Andean and Sub-Andean areas of southwestern Bolivia (from La Paz Department to Tarija Department), northern Chile (Arica- Parinacota and Tarapacá regions), and northwestern Argentina (several localities in Salta and Jujuy provinces, one in La Rioja Province, being highly probably in Catamarca Province—Cabrera 1961; Mann Fischer 1978; Pine et al. 1979; Contreras et al. 1987; Mares et al. 2000; Muñoz-Pedreros 2000; Díaz and Barquez 2002; Díaz and Verzi 2006; Díaz and Barquez 2007; Verzi et al. 2015a; Rivera et al. 2016). In Argentina, this species occupies the High Andes, Monte Desert of Mountains and Isolated Valleys, and Puna ecoregions (Díaz and Verzi 2006). Ojeda et al. (2011) mentioned that O. gliroides is restricted to northern Monte of Mountains and Isolated Valleys. It is found on both sides of the Andes, a distribution marked by an east to west gradient of decreasing rainfall that results in east and west (from theAndes) populations with contrasting differences in the abundance and patchiness of vegetation cover ( Rivera et al. 2014, 2016).
This species has an elevation range from about 1,200 m at Villa Union in La Rioja Province, Argentina to about 4,400 m in Potosí, Bolivia. Verzi et al. (2015a) indicated that the lowest altitude for the distribution of this species is 200– 300 m. However, this information is incorrect because the data taken from Diaz and Ojeda (1999) correspond to measurements of precipitation (200–300 mm), and this information was misinterpreted by editors during the final process of editing the book Mammals of South America (Patton et al. 2015).
FOSSIL RECORD
Although Octodontomys gliroides has no fossil record, it is the most primitive extant octodontid. Its cranial and dental anatomy resemble some species from the Miocene and Pliocene of Argentina (Verzi and Carrín Iglesias 1999; Verzi et al. 1999; Verzi 2002; Verzi et al. 2014), suggesting that it may be an early octodontid offshoot ( Verzi et al. 2015b). Gallardo and Kirsch (2001) mentioned that Verzi (1994) considered hypsodonty in Ctenomys and Octodontomys to be indicative of common ancestry and re-erected the subfamily Ctenomyinae to include these 2 genera; however, their interpretation of Verzi’s conclusion was erroneous because Verzi stated “hypsodonty has originated independently several times in rodents” (see Verzi 1994:92) and thus does not support the idea of common ancestry.
Opazo (2005) estimated the divergence of Octodontomys from its sister clade ( Octodon + Spalacopus + Aconaemys ) at 6.07 ± 1.34 million years ago, near the end of the Miocene. This value agrees with the results of both Gallardo and Kirsch (2001), who used DNA hybridization data, and Verzi et al. (2016), using morphological and molecular data, to obtain an estimated divergence of 5–7 million years ago; but it is older than the estimate of Honeycutt et al. (2003; 2.9–4.1 million years ago) based on molecular data (nucleotide sequence data from nuclear receptor and mitochondrial genes nucleotide sequence).
The morphology of the lower incisor is shared with the extinct octodontid Neophanomys , but whether this character state is primitive or derived is difficult to determine (Verzi et al. 1999). Octodontomys has been recorded in the archaeological site Inca Cueva 5 site, Jujuy Province, Argentina (2,120 ± 120 to 780 ± 100 years before present) based on 3 left mandibular rami; the area corresponds to the oriental border of the Puna steppe (Teta and Ortiz 2002).
Octodontomys gliroides produces middens (amalgamations of plant remains, bones, insects, feathers, and rodent feces, glued together within a crystallized matrix of rodent urine), which have proven to be important sources of paleontological evidence (Betancourt and Saavedra 2002; Latorre et al. 2005). In the Atacama ( Chile), middens have been recorded from possibly the Quaternary for O. gliroides as well as other families of rodents (e.g., Chinchillidae , Abrocomidae , and Muridae—Latorre et al. 2005).
Arnal and Vucetich (2015a, 2015b) used extant species of the family Octodontidae , including O. gliroides , to revise the taxonomy of the fossil rodent Acaremys ( Hystricognathi , Octodontoidea, Acaremyidae ) and to analyze phylogenetic relationships of the Pan-Octodontoidea group.
FORM AND FUNCTION
Dental formula for Octodontomys gliroides is i 1/1, c 0/0, p 1/1, m 3/3, total 20. Buccal surfaces of the upper teeth are progressively rotated more distally from the distal to the mesial ends of the arches, with DP4 being nearly transverse to the line of the arch (Miles and Grigson 1990). O. gliroides has simplified teeth without reentrant angles or cement (Glanz and Anderson 1990). In hypsodont rodents, the enamel of the molariforms typically has a different structure on the side of first contact (leading edge) and the opposite face (trailing edge); O. gliroides is more primitive compared to Ctenomys in this regard, with Hunter– Schreger bands limited to some areas.
In a study of ultrastructure of the incisor enamel and delineated morphofunctional traits among octodontoid genera with disparate digging adaptations, O. gliroides had an enamel thickness more than double that of the other taxa studied (e.g., Ctenomys , Octodon , Dactylomys — Vieytes et al. 2007). The relative thickness of external index showed the lowest values in the fossorial O. gliroides and the arboreal Bolivian bamboo rat Dactylomys boliviensis than the other species studied, and this has been interpreted by Vieytes et al. (2007) as indicative of low wear resistance.
The middle ear of O. gliroides has 3 cavities: bulla, anterior, and posterior recesses. The bulla is divided into 8–10 large pneumatized air cells, and the cochlea protrudes into the tympanic cavity medially ( Heller et al. 1976). In the normal ear, these structures have fairly uniform radiolucency without evidence of thickening of the septae of the bulla or areas of bony sclerosis ( Heller et al. 1976).
In extant species of fossorial rodents, the degree of reduction in size of the eye indicates the adaptation level to the subterranean lifestyle ( Pearson 1984). The size of the orbital cavity provides information about the size of the eye and can be estimated using the zygomatic index (see Verzi 2002:316). The comparative analysis of Verzi (2008) showed that echyimid rodents have relatively larger orbits than octodontids (for O. gliroides , mean index value of 1.22, range 1.13–1.38). The eyes of O. gliroides are much larger than those of Octodon , matching the inferred crepuscular or nocturnal habits of this species ( Rowlands 1974, not seen cited in Wilson and Kleiman 1974) compared to the apparently diurnal habits of Octodon (Wilson and Kleiman 1974) .
Vasallo and Mora (2007) included O. gliroides in an allometric analysis of the effect of size on skull attributes (development of the mandibular angle and masseteric crest, and the robustness of incisors) in ctenomyid and octodontid rodents. They concluded that in Octodontomys , as in other octodontids and in ctenomyids, mandibular width and cross-sectional shape of the incisors show a significant and positive association with basicranium length. They also noted that O. gliroides clearly differs from Ctenomys by the intensification of the hystricognath condition (lateral expansion of the mandibular angle and masseteric crest) in Ctenomys .
The glans penis of Octodontomys has 2 long spines on each side of the intromittent sac (2-2 pattern), although some specimens show a 2-3 pattern ( Spotorno 1979; Contreras et al. 1993); these spines are the longest among octodontids (average length 5.6 mm—Contreras et al. 1993). These features indicate that O. gliroides is more closely related to generalist and subterranean species than to Octomys or Tympanoctomys , as the 1-1 pattern is interpreted to be ancestral and the 2-2 pattern derived in extant octodontid rodents ( Contreras et al. 1993). O. gliroides shows the longest baculum among octodontids, which gradually tapers from the base to the tip ( Contreras et al. 1993). The vaginal wall of O. gliroides shows a hardened surface and does not have a complementary space to hold the spikes in a distal or lateral position, suggesting that they can be accommodated only when pointing backwards ( Contreras et al. 1993).
Cummins and Woodall (1985) offer the sperm dimension of O. gliroides as follows: head length, 5–29 µm; head width, 6–50 µm; midpiece length, 5–32 µm; principal length, 34–57 µm; and total length, 45– 18 µm. The maximum duration of ejaculatory mounts recorded in O. gliroides was 60 s ( Kleiman 1974).
Bozinovic (1992) determinated the body mass and basal metabolic rates of grazing rodents from Chile and noted a body mass of 156.3 ± 3.0 g for O. gliroides , a basal metabolic rate of 0.805 ± 0.022 ml O 2 g−1 h−1, and a proportion of predicted basal rate of metabolism of 94.3%. The thermoneutral zone of O. gliroides extends from 25°C to 35°C, within which 2 individuals had a basal rate that was 105% of the value predicted from body mass; at an ambient temperature of 25°C, they had 90% of the value expected from body mass.At temperatures between 5°C and 34°C, the mean body temperature was 37.2 ± 0.08°C; below 4°C, an animal became hypothermic, an unexpected observation given the harsh natural environment in which O. gliroides lives. Correlated with this breakdown in temperature regulation was a decrease in metabolic rate (Arends and McNab 2001).
Octodontomys gliroides has less-developed renal papilla, low renal indices, and low urine concentration in comparison with the plains viscacha rat Tympanoctomys barrerae , reflecting a water-rich diet of cacti (Diaz and Ojeda 1999). The data presented by Diaz and Ojeda (1999) for O. gliroides are: cortex thickness, 1.83mm; medullary thickness, 6.83 mm; relative medullary thickness (RMT = ratio of 10 times the medullary thickness to the cube root of the product of length × width × thickness of the kidney), 5.35; ratio of inner medulla cortex, 3.19; relative medullary area, 1.03. The RMT is considerably lower than values for other desert rodents, such as the plains viscacha rat (Diaz and Ojeda 1999). In Chile, Al-kahtani et al. (2004) obtained a RMT of 8.5 and a kidney mass of 1.72 g for O. gliroides ; they determined that rodents from arid areas have the largest RMT values and, through a phylogenetic analysis, that mass-corrected kidney mass is positively correlated with habitat aridity.
The longevity of 1 specimen in captivity was 7.6 years ( Weigl 2005). Molting was observed in specimens captured in February, March, June, and December (Díaz and Barquez 2007). The digestive tract has a well-developed colon and cecum (Muñoz-Pedreros 2000).
ONTOGENY AND REPRODUCTION
The age at sexual maturity of Octodontomys gliroides is 5 months (Ralls and Ballou 1982). The gestation period, in captivity, is 99–104 days with a litter of 2–4 offspring; the young are precocial and born completely furred with open eyes, weighing 15–21 g at birth, and lactation lasts 5–6 weeks ( Weir 1974; Wilson and Kleiman 1974; Redford and Eisenberg 1992). Puberty is indicated by the perforation of the vaginal closure membrane; it has been observed in O. gliroides at about 3–4 months (Wilson and Kleiman 1974).
In a study developed in Jujuy Province, Argentina, Díaz and Barquez (2007) recorded in February, 1 female with a closed vagina, 1 lactating female with 6 mammae (2 axillary, 2 abdominal, and 2 inguinal), 1 lactating female, and 1 female with 2 fetuses; in March, they recorded females with closed vaginas. Males with abdominal testes were recorded in both February and March, and males with scrotal testes in December. Juveniles were captured in February and November, and births were recorded in February and March, so juveniles of different ages were present in the population at the same time of the year in the same location. In Chile, a lactating female and a juvenile were mentioned by Pine et al. (1979) during November. In Bolivia, young were recorded in January and May, and pregnant females were recorded in September (2 embryos) and October (1 embryo— Anderson 1997).
ECOLOGY
Octodontomys gliroides is a typical species of dry areas. It lives in small burrows among rocks or cactus roots, with scattered vegetation, shrubs, and cacti ( Thomas 1913; Walker 1964; Ipinza et al. 1971; Díaz and Barquez 2007), where it feeds on vegetation, hides from terrestrial and aerial predators, and rears offspring ( Rivera 2013). In an ecological study of Leopardus jacobita in the Andean region of Jujuy Province ( Argentina), O. gliroides was cited as an important food item of this Andean mountain cat because of their biomasses and relatively high frequencies ( Reppucci 2012).
The openings of its burrows are connected by superficial tracks, and all the vegetation around the burrow is removed except thorny species ( Ipinza et al. 1971). In Chile, the vegetation where O. gliroides is distributed is dominated by the cacti Browningia candelaris , Cereus atacamensis, Polycereus , and Opuntia as well as shrubs and herbs such as Polyachyrus tarapacanus , Viguiera gayana , Piqueria pinifolia , Psila boliviensis , Trixis cacaloides , and Mentzelia ignea (Muñoz-Pedreros 2000) . According to some authors, O. gliroides is nocturnal (e.g., Ipinza et al. 1971; Wilson and Kleiman 1974; Mares and Ojeda 1982), whereas other authors consider it diurnal ( Thomas 1913; Mann Fischer 1978; Díaz and Barquez 2007). Díaz and Barquez (2007) mentioned that it may remain active during the first hours of darkness. On the other hand, Rivera et al. (2014) used radiotelemetry to determine that the females and males were more active during nighttime than daytime.
In Jujuy ( Argentina), O. gliroides was captured with other mammalian species such as the white-bellied akodont Akodon albiventer, Andean mouse Andinomys edax , common yellowtoothed cavy Galea musteloides (now Galea leucoblephara ), yellow-rumped leaf-eared mouse Phyllotis xanthopygus (Rodentia) , and the white-bellied fat-tailed mouse opossum Thylamys pallidior (Didelphimorphia—Díaz and Barquez 2007) .
Based on an isotopic study of hair from animals inhabiting the Andean region of Jujuy Province ( Argentina), it was determined that O. gliroides represents the first active link of the local food chain (Panarello and Fernández 2002). It is an herbivorous species with a diet rich in cellulose, with the composition of its diet changing from cacti in the winter to an apparently graded mixture of CAM (Crassulacean acid metabolism) species with a proportion of up to 30% of C3 grasses during the summer; it uses the watery tissues of the cacti Cereus and Opuntia as water source (Muñoz-Pedreros 2000; Panarello and Fernández 2002). Other plants eaten by O. gliroides included: Maihueniopsis glomerata , M. boliviana, Tarassa , Gnaphalium lacteum , and Senecio graveolens (Panarello and Fernández 2002) .
This species can suffer from otitis caused by Pseudomonas aeruginosa ; clinically, the tympanic membrane varied from mild hyperemia to dull opacification with collection of fluid behind it; radiographically, an increase in radiodensity and thickening of the wall and septa of the bulla characterize the infection ( Heller et al. 1976). Trypanosoma cruzi , the hemoflagellate responsible for Chagas disease, has been recorded in specimens of O. gliroides . It is thought that O. gliroides may have been involved with the domestic cycle of transmission with humans since prehistoric times ( Schweigmann et al. 1992). Recently, Buitrago et al. (2016), in a study developed in Bolivia, mentioned that O. gliroides is the predominant blood meal sources of wild populations of T. infestans (36%) and also revealed that there may be links between wild and domestic cycles of the T. cruzi transmission.
Regarding ectoparasites, O. gliroides hosts fleas of 2 species of the genus Ectinorus : E. nomisis in Argentina and Chile and E. simonsi in Bolivia and Chile ( Smit 1987; Beaucournu et al. 2013, 2014). It has been suggested that E. nomisis should likely be regarded as a subspecies of E. simonsi ( Beaucournu et al. 2013) . Other species of fleas reported on O. gliroides are: Neotyphloceras crassispina hemisus , Nonnapsylla rothschildi , and Hectopsylla suarezi ( Beaucournu et al. 2014) . A louse of O. gliroides , Ferrisella disgrega ( Phthiraptera, Anoplura ), has been recorded from Zapahuira and Chuzmiza ( Chile), Oruro ( Bolivia), and Jujuy (Argentina—Castro and Cicchino 1987 as Hopopleura disgrega ; Durden and Musser 1994 as H. disgrega ; Castro and Verzi 2002; Moreno Salas et al. 2005).
BEHAVIOR
Octodontomys gliroides has not been observed in a family group; it is likely that the male is not bound to the same burrow as the female, since O. gliroides is less social than the highly communal common degu Octodon degus ( Rowlands 1974 in Wilson and Kleiman 1974). Rivera et al. (2014) determined that ecological differences (abundance and distribution of food, predation risk, and burrowing costs) in 2 populations of O. gliroides did not translate into social differences (group size and range area), and they also indicated that sociality of O. gliroides had an ancient origin.
In O. gliroides , mutual upright body position with incisor interlocking and riding has been observed as part of social play; all members of families of O. gliroides also frequently sandbathed at the same location, often one after the other, and locomotor-rotational movements have also been observed (Wilson and Kleiman 1974). According to Emilio Budin in Thomas (1913:143), the specimens “are not wild, and are easily shot.” In captivity, family groups (parents and young) immediately engage in intense social activity after awakening and do not eat until much later; this suggests that family groups may play together close to the nest site and then disperse to forage in nature (Wilson and Kleiman 1974).
In O. gliroides , a pre-copulatory vocalization, a modified version of the alarm squeak, and gurgle or twitter similar to those produced by some birds have been reported ( Eisenberg 1974; Kleiman 1974). It emits low and middle frequency calls, and also several high frequency or ultrasonic vocalizations ( Eisenberg 1974), although the range of vocalizations of subterranean rodents is predominantly of low frequency ( Schleich et al. 2007).
GENETICS
Octodontomys gliroides has a diploid number (2n) of 38 chromosomes and a fundamental number (FN) of 64; metacentric and submetacentric chromosomes represent 60% of the karyotype, with the rest comprised of acrocentric and subacrocentric chromosomes (George and Weir 1972). The karyotype of O. gliroides seems to be an extremely derived one, differing from those of all other octodontid species ( Contreras et al. 1994). The extreme variation observed in chromosome number (2n = 38–102) among octodontids ( Contreras et al. 1990; Gallardo 1992; Spotorno et al. 1995) has been interpreted as resulting from chromosome fusions, with lower numbers derived from ancestral karyotypes with greater numbers ( Spotorno et al. 1988). Although a bidirectional trend with a decrease from the modal 2n = 56–58 observed in O. gliroides (2n = 38) and a saltational increase observed in the plains viscacha rat (2n = 102) has also been proposed ( Gallardo 1992; Gallardo 1997).
C-banded chromosomes reveal the presence of pericentric heterochromatin on the X chromosome and 6 autosomal pairs ( Contreras et al. 1994). However, for animals from the same locality, Gallardo (1992) reported that the sex chromosomes were totally heterochromatic, and according to Contreras et al. (1994), cannot be attributed easily to technical differences. Moreover, if a consequence of the heterochromatinization is gene inactivation, this condition, affecting the entire X chromosome, would be the most extreme condition reported for any mammal ( Contreras et al. 1994). A hybridization analysis under relaxed conditions demonstrated that RPCS (repetitive PuvII Ctenomys sequence)-related sequences were present in O. gliroides , which is suggested to have diverged from the genus Ctenomys more than 10 million years ago ( Rossi et al. 1990). However, there is a striking difference between the tandem repeats of RPCS of O. gliroides and those of Ctenomys ( Rossi et al. 1990) . O. gliroides has been referred to both the Ctenomyinae and the Octodontinae ( Verzi 2001) , but molecular data provide no evidence for excluding O. gliroides from Octodontidae sensu stricto (Gallardo and Kirsch 2001); as Verzi (1994) suggested, the craniomandibular and dental morphology ( Verzi 2001) and penial morphology ( Contreras et al. 1993) may demonstrate high levels of homoplasy ( Gallardo et al. 2007).
The phylogenetic position of Octodontomys remains uncertain, but in the analysis ofUpham and Patterson (2012) combined 4-gene data set, this genus is positioned basal to the desert-adapted clade, sister to Octomys + Tympanoctomys ; whereas in the same work a BEAST analysis recovered Octodontomys as sister group of Octodon ( Spalacopus, Acoanemys ). Upham and Patterson (2015) with the inclusion of a 5th gene data set (cytochromeb) recovered in all the analysis the same topology as Honeycutt et al. (2003) who represented a clade by placed Tympanoctomys and Octomys , grouped separate from Octodontomys , which was sister to a clade containing Octodon and a clade represented by Aconaemys and Spalacopus (also see Gallardo and Kirsch 2001; Opazo 2005; Verzi et al. 2015a, 2016). Gallardo et al. (2003) found significant differences in DNA content within the family Octodontidae , with Octodontomys differing significantly from the genera Spalacopus , Octomys , and Aconaemys . In addition, these authors indicated a time of divergence to a form split from the common degu Octodon degus that gave rise to O. gliroides during the Late Miocene, about 5.99 million years ago; in concordance with Gallardo and Kirsch (2001). Rivera et al. (2016) based on Bayesian analysis of mtDNA control region haplotypes recovered O. gliroides as a well-supported monophyletic group composed of 2 principal lineages, namely lineages A and B. Lineage A was widespread in Bolivia and Chile, and comprised 20 haplotypes in 93 individuals from populations in northern, central, and southern Bolivia, and northern Chile. While lineage B was geographically restricted mainly to Argentina, including 6 haplotypes in 6 individuals and 1 haplotype in a single individual belonging to the southern Bolivian population.
Ctenomys and Octodontomys are the only vertebrates where shorter S rRNA have been reported; the splitting or elimination of the intron has not been found in other rodents, indicating horizontal acquisition in the common ancestor of Ctenomys and Octodontomys ( Melen et al. 1999) .
CONSERVATION
Octodontomys gliroides is common in suitable habitat, and no major threats are known to this species. It is considered a species of “Least Concern” (Diaz and Ojeda 2000; Muñoz-Pedreros 2000; Tirira et al. 2008; Ojeda 2012).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Octodontomys gliroides (P. Gervais and
| Pérez, M. Julieta & Díaz, M. Mónica 2018 |
Octodontomys gliroides
| : Thomas 1913: 143 |
Octodontomys gliroides
| : Thomas 1913 |
Neoctodon simonsi
| Thomas 1902: 115 |
Octodon gliroides P. Gervais and d’Orbigny, 1844:22
| P. Gervais and d'Orbigny 1844: 22 |