Haritalodes derogata (Fabricius, 1775)
|
publication ID |
https://doi.org/10.25221/fee.465.3 |
|
publication LSID |
lsid:zoobank.org:pub:250E2D3C-0535-4BFC-A103-0B302D39B49D |
|
persistent identifier |
https://treatment.plazi.org/id/0397D80D-C366-FFE9-FF7D-FAE6FE32FE48 |
|
treatment provided by |
Felipe |
|
scientific name |
Haritalodes derogata (Fabricius, 1775) |
| status |
|
Haritalodes derogata (Fabricius, 1775) View in CoL
Figs 1–4 View Fig View Fig View Fig View Fig
MATERIAL EXAMINED. Russia: Sochi , Nagornaya str., 09.VII 2021, larvae from
Hibiscus syriacus , 7♂, 9♀, adults emerged 10-15.VIII 2021, N. Karpun, E. Zhuravleva, E.
Shoshina coll.; Sochi, Yana Fabritsiusa str., 12-13.VIII 2021, larvae from H. syriacus , 14♂,
17♀, adults emerged 23-29.VIII 2021, 1 larva DNA barcoded (Sample ID: NK1072; Process
ID: CAMRU157-21 ), E. Zhuravleva, E. Shoshina coll.; ibidem, 13.VIII 2021, larvae from
Abutilon hybridum , 4♂, 5♀, adults emerged 23-30.VIII 2021, N. Karpun, E. Zhuravleva,
coll.; ibidem, 15.VIII 2021, larvae from H. syriacus , 4♂, 5♀, adults emerged 25-30.VIII 2021,
N. Karpun, E. Zhuravleva, coll.; Sochi, park “Southern Cultures”, 08.VIII 2021, larvae from
Tilia caroliniana , 5♂, 6♀, adults emerged 20-27.VIII 2021, 1 larva DNA barcoded (Sample
ID: NK1071; Process ID: CAMRU 156-21), N. Karpun, E. Zhuravleva, N. Kirichenko coll.;
Sochi, Zeleny alleyway, 14.VIII 2021, larvae from H. syriacus , 4♂, 5♀, adults emerged 31.VIII
2021, E. Zhuravleva coll.; ibidem, same collection date, larvae from A. theofrasti , 3♂, 4♀,
adults emerged 31.VIII-5.IX 2021, E. Zhuravleva coll.; Sochi, Lazarevskoe, 29.VIII 2021,
larvae from H. syriacus , 5♂, 5♀, adults emerged 12-19.IX 2021, E. Zhuravleva, E. Shoshina coll.; ibidem, 13.VIII.2022, larvae from A. hybridum , 1♂, 2♀, adults emerged 23-30.VIII
2022, E. Zhuravleva; Sochi, Kurortniy av., 21.VIII 2021, larvae from H. mutabilis , 5♂, 5♀,
adults emerged 05-11.IX 2021, E. Zhuravleva, E. Shoshina coll.; Sochi, Gagarina str., 28.VIII
2021, larvae from A. hybridum , 4♂, 5♀, adults emerged 10-16.IX 2021, E. Zhuravleva, E.
Shoshina coll.; ibidem, Gagarina str., 08.VIII 2022, larvae from T. begoniifolia , 5 larvae, 1
pupae, E. Zhuravleva coll.; Anapa, Shevchenko str., H. syriacus , 07.IX 2022, 1 pupa, E.
Shoshina coll.
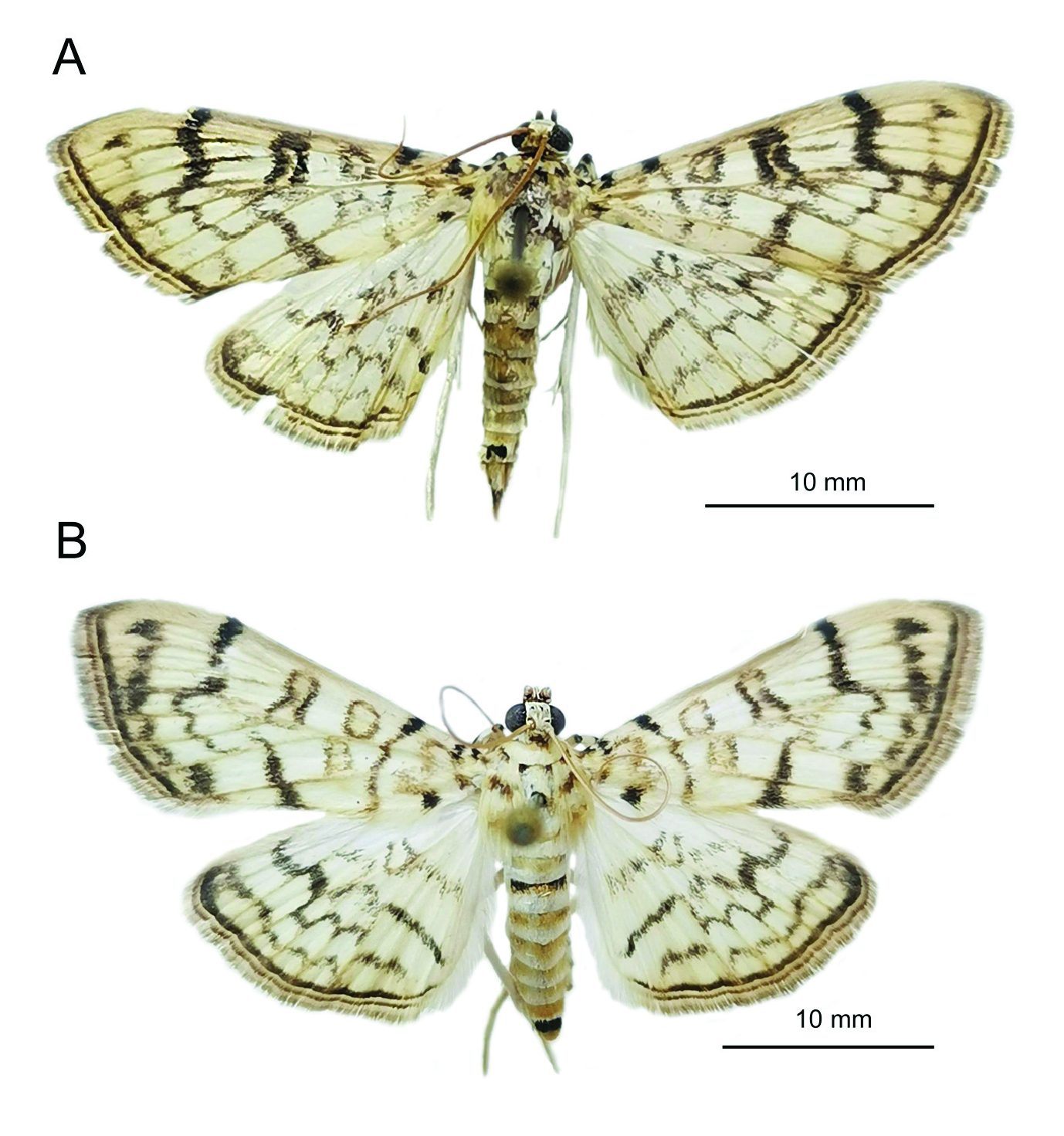
ADULTS. The morphology of the specimens collected in Sochi ( Fig. 1 View Fig ) well corresponds to the detailed description of the moth habitus given in Kumar et al. (2013). The body length of the specimens from Sochi was 12,6±0,05 ( ♂) and 13±0,03 ( ♀) mm, the wingspan 25,6±0,18
( ♂) and 27,4±0,14 ( ♀) mm.
syriacus, Sochi, Yana Fabritsiusa str., 25.VIII 2022 (the adult emergence date).
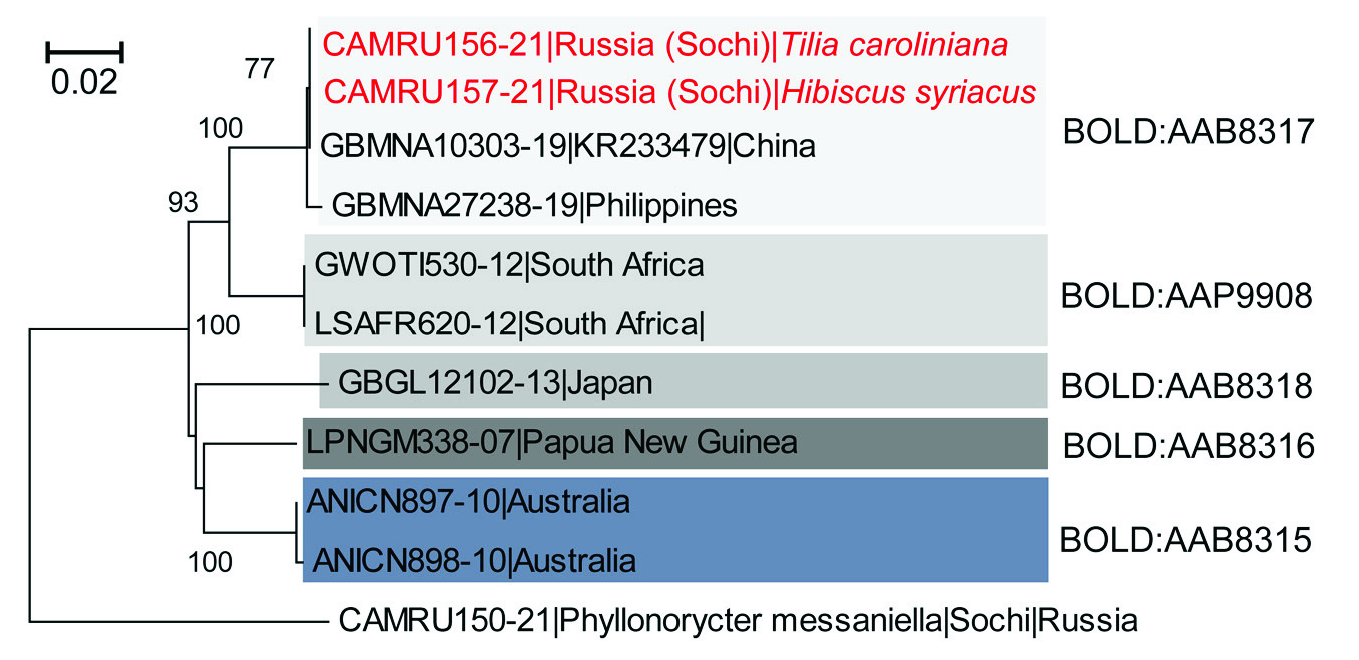
DNA BARCODING. DNA barcoding data obtained for two larvae sampled from H.
syriacus and T. caroliniana in Sochi ( Russia) allowed identifying H. derogata with 100%
confidence in BOLD, where the closest neighbor originated from China ( Fig. 2 View Fig ). No divergence was recorded between two specimens from Sochi. They, together with the specimen from China, formed one cluster with the specimen from Philippines (0,47% divergence) corresponding to one BIN: BOLD: AAB8317 ( Fig. 2 View Fig ; Table 2). Interestingly, the other specimens identified as H. derogata in BOLD and/or GenBank formed four other divergent clusters, with each cluster corresponding to a different BIN. Among them, two specimens from South Africa were assigned to BIN: BOLD: AAP9908 and two specimens from Australia to BIN: BOLD: AAB8315 ( Fig. 2 View Fig ). The specimens from Japan and Papua New Guinea corresponded to the BINs: BOLD: AAB8318 and BOLD: AAB8316 accordingly ( Fig. 2 View Fig ) .
derogata specimens originating from different countries.
Remark. Minimal pairwise distances are given for each species pair; values in square brackets represent maximal intraspecific distances; [—] no data because a single specimen was sequenced.
Minimal pairwise distances estimated for each species pair varied from 4,15% (for the specimen from South Africa and Russia (Sochi) to 7,36% (for the specimens from South Africa and Japan) ( Table 2). Such pronounced genetic distances between the specimens of H. derogata originating from different parts of the world may suggest the presence of cryptic species.
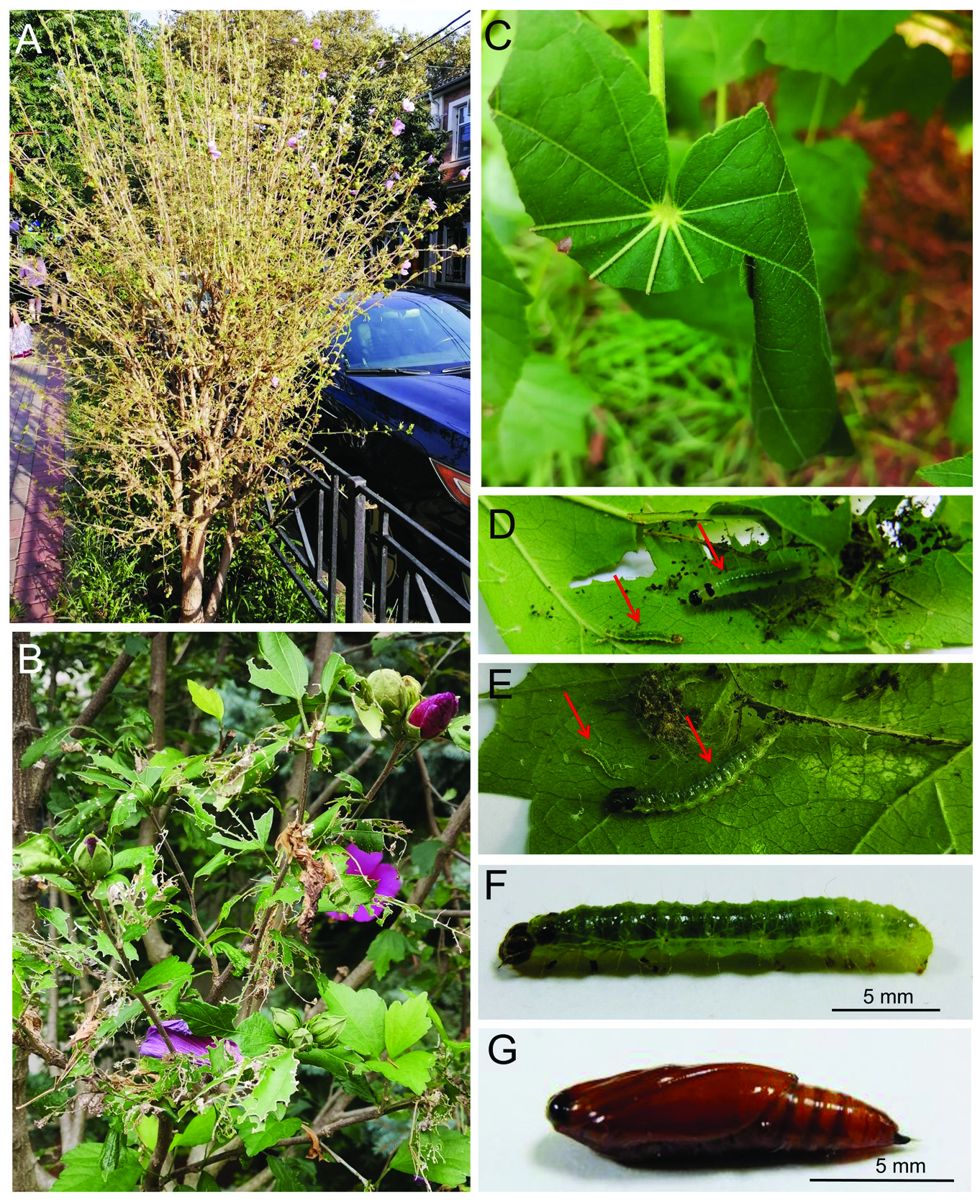
BIOLOGY. In Sochi, the larvae caused characteristic damage to the leaves ( Fig. 3A–C View Fig ).
Larvae made long cut in the upper part of the leaf blade and rolled the cut part downwards
( Fig. 3C View Fig ) binding the roll with silk. Larvae fed inside such constrictions during whole larval stage. At the beginning, they stayed together (i.e. the larvae from the 1st to the 5th instar)
inside a leaf roll; later they dispersed on a host and fed singly in new leaf rolls ( Fig. 3 View Fig D-F).
Young larvae skeletonized, the older ones rudely gnawed leaf lamina inside rolled leaves.
Matured larvae left their shelters and made new leaf rolls in which they pupated. Matured larva were 25–27 mm long. Pupae were 13-15 mm long, brown, cremaster with an awl-shaped projection ( Fig. 3G View Fig ).
The pest is commonly known to develop three generations; exceptionally, in warmer regions, up to four generations were documented (Dhindsa et al., 1980; Uematsu, 1986). In the humid subtropics of the Black Sea Coast in 2021–2022, two generations were recorded per year: after overwintering, adults emerged in the second half of June, whereas the adults of the second generation were emerging during most of August. Adults lived 7-10 days. They were active in late evening and night and were attracted by the light. Outdoor, the larvae of the first generation pupated inside the rolled leaves on plants, whereas the larvae of the second generation pupated in rolled leaves on the litter or under the bark of host plants.
Under natural conditions, the entire life cycle of one generation took 30–42 days. In laboratory conditions, the females laid up to 300 eggs singly on the lower surface of host plant leaves. The eggs were small, greenish, elliptical. Under laboratory conditions, larval stage took about 20 days, pupation took 8-10 days. These data are consistent with the biology of H. derogata described in other countries, in particularly, India and Azerbaijan (Badiyala,
2011; Sontakke et al., 2015; Gahramanova et al., 2020).
derogata in its modern range. The clusters are indicated by different colors correspond to different BINs. Each specimen is indicated by the BOLD process ID (begins with CAMRU.
GBMNA etc.), followed the country of origin (and the locality, in case of Russia), and a host plant (where known). Bootstrap values> 70 are given next to the corresponding branches.
HOST PLANTS: Polyphagous species on Malvaceae , Fabaceae , Rhamnaceae , Anacar-
diaceae, Amaranthaceae , and Rosaceae . In South East Asia, Africa and Oceania, larvae feed on leaves of Hibiscus , Gossypium , Sida , Pavonia , Anacardium occidentale , Glycine max ,
Zizyphus mauritiana , Z. jujuba , Abelmoschus esculentus , and others (Mariselvi & Manimegalai, 2016; Roychoudhury et al., 2017). In the Russian Far East, larvae feed on herbaceous,
subshrubs, and woody plants from several families (Sinev & Streltsov, 2019).
On the Black Sea Coast of Russia, Haritalodes derogata was initially recorded in plantings of Hibiscus syriacus . Further surveys of 2021 and 2022 showed that it also attacks
H. sinensis , H. mutabilis , H. lasiocarpos in all districts of Sochi. In the beginning of August
2021, H. derogata larvae were documented on the Tilia caroliniana and T. begoniifolia in the
Imeretinskaya Lowland (the dendropark “Southern Cultures”). In late August 2021, larvae were found also on Abutilon × hybridum in ornamental plant, and on Tilia platyphyllos . In the same period, the characteristic damage was recorded on the weedy plant Abutilon theophrasti .
2021. А – severe damage caused to Hibiscus syriacus ; B – close up of the damaged plant;
C – characteristic leaf damage; D, Е – unrolled leaf with young and late instar larvae
(indicated by red arrows) and black frass; F – larva of the 5th instar (lateral view); G – pupa
(lateral view).
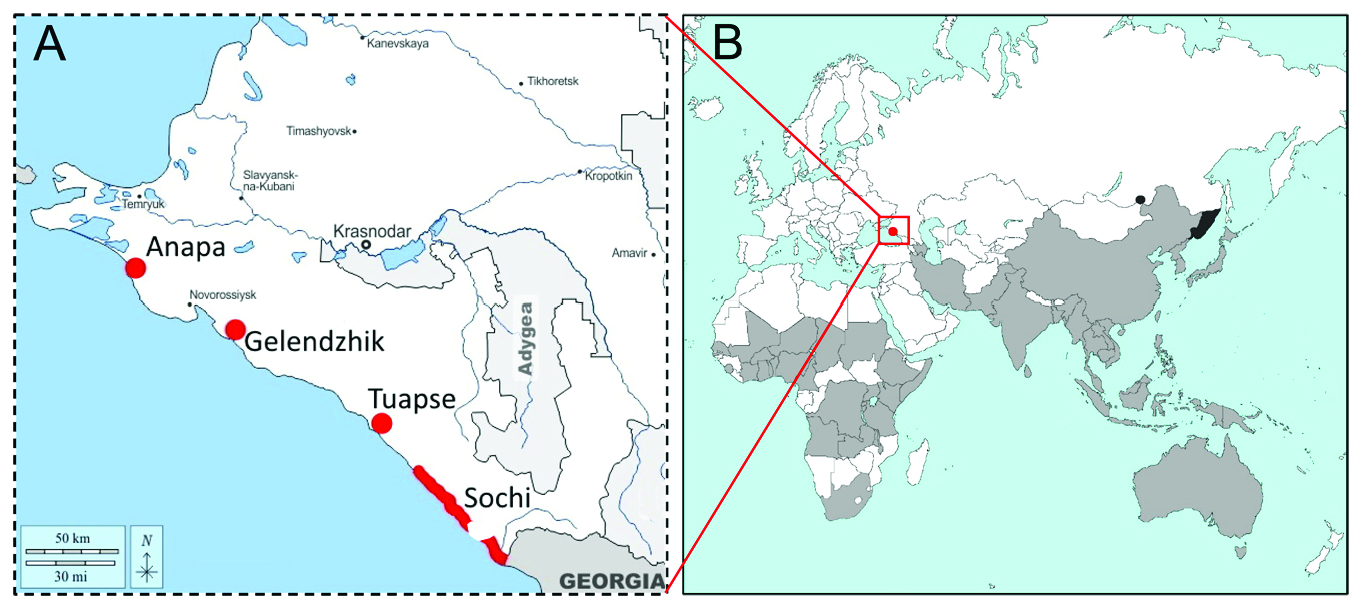
DISTRIBUTION. Africa, Asia, Oceania ( Fig. 4 View Fig ); the detailed list of the countries where the pest occurs is given in Byun at al. (2008) and CAB International (2022). Russia: South
Primorye (Bremer, 1864), Transbaikalia (Korsun, 2017), Sochi (discovered in July 2021)
(present paper), Gelendzhik ( September 2021) (Krylenko, 2022), Anapa, Tuapse, Sochi
(Krasnaya Polyana, 960 m above sea level) ( September 2022) (present paper).
derogata was discovered in 2021–2022 (A) and the modern range of the species (B). Present distribution of H. derogata is shaded in gray; the regions colored in black show the presence of the species in Russia, the red dot indicates the recent species invasion to the South of Russia.
DAMAGE LEVEL. In summers 2021–2022, the species caused severe damage to Hibiscus
spp. in Sochi: the defoliation level reached nearly 100% in Yana Fabritsiusa str. and Zeleniy line. In the other localities, the damage level was around 50%. The most damage was caused in the end of July – early August. The bushes of hibiscus severely defoliated in 2021,
withstood and refoliated next summer 2022. In July–August 2022, hibiscus bushes experienced similar level of damage as the treatment procedures were delayed. In a contrast to hibiscus, the damage to Tilia spp. was occasional and not intense (less than 25% of leaves were damaged in the dendropark “Southern Cultures” and Centralniy district of Sochi).
CONTROL MEASURES. Chemical control is used in extreme cases to localize outbreaks in the regions of cultivation of cotton, soybean, cashew, and other crops. The insecticides based on thiomethoxam, imidacloprid, cypermethrin, lambda-cyhalothrin, and dimethoate showed satisfactory efficiency (Misra et al., 2002; Badiyala, 2011; Silvie et al., 2013;
Shakya & Saxena, 2016). Due to many restrictions, the use of insecticides in ornamental plantings, especially in the resort area (such as Sochi), is very limited or impossible. Presently,
no effective cure is developed to control the pest populations on Russian Black Sea Coast.
There are data on parasitoids from the families Braconidae , Ichneumonidae and Eulophidae attacking larvae and pupae of H. degorata , and larval pathogens, i.e. Bacillus thuringiensis var. Kurstaki and Beauveria bassiana (Odebiyi, 1982; Kumar et al., 2013; Gahramanova et al., 2020; Dannon et al., 2021).
PROGNOSIS. In the absence of effective measures by the relevant authorities, the forecast of further expansion of H. derogata in the Southern Russia cannot be optimistic. The two-
year observations run in Sochi suggests a high risk of H. derogata invasion across Southern
Russia. Furthermore, the accidental introduction of this polyphagous pest to the European regions with the subtropical climate and its rapid acclimatization with following damage to crops and ornamental plants are not ruled out.
CONCLUSIONS. The vector of H. derogata invasion to the Russian Black Sea Cost remains unknown. We suspect that the introduction of the pest to this region could be human-mediated. The species could be brought here accidently with plants for planting or transported with other goods. Bearing in mind that the pest already provides noticeable damage in Sochi, it has no doubts that it penetrated the region some years ago. As a matter of fact, it was overlooked here and detected only when the damaged became pronounced.
Urgent actions are required to localize the foci of the alien pest species on the territory of the Russian Black Sea Coast and prevent its further dispersal to neighboring regions and countries. Further studies would be needed to explore the genetic diversity of H. derogata across its wide range in Asia, Africa and Oceania to resolve the question about the existence of sister species, as well as determine the regions from where the pest could be introduced to the Black Sea Cost of Russia. These data would be of a high importance to understand the invasion history, define the distribution routes and develop the adequate management methods for controlling the pest.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.