Parastacus brasiliensis promatensis, Fontoura, Nelson Ferreira & Conter, Maria-Rotraut, 2008
|
publication ID |
https://doi.org/ 10.5281/zenodo.183370 |
|
DOI |
https://doi.org/10.5281/zenodo.5625773 |
|
persistent identifier |
https://treatment.plazi.org/id/0398A275-FFBF-FF98-FF73-238D49FE5404 |
|
treatment provided by |
Plazi |
|
scientific name |
Parastacus brasiliensis promatensis |
| status |
subsp. nov. |
Parastacus brasiliensis promatensis View in CoL subsp. n. ( Fig. 1)
Type material. Holotype: MCT 2085. 1 specimen, Brazil, Rio Grande do Sul, São Francisco de Paula, Pró- Mata, Garapiá stream (29o29.371’ S; 50o13.800’ W), 22.xi.1997, Leg. M. Conter. Paratypes: MCT 2086. 8 specimens, same location as holotype.
Etymology. The subspecies was named after the only location from where it is known. Diagnostic characters. Identification should be based on a set of morphometrical data ( Tables 2 View TABLE 2 and 3 View TABLE 3 ) as there is no morphological character that clearly distinguishes P. brasiliensis promatensis from P. brasiliensis brasiliensis .
Description. Carapace laterally compressed, surface smooth, with small pores and scattered hairs. Subtriangular rostrum, usually extending up to the second segment of the antennula. Rostrum surface flat, edged by crests that converge to the apex, ending in a short and depressed spine. Long hairs, growing down to the orbit from the rostral margins. The aureole is narrower at the centre, with branchial crests anteriorly and posteriorly diverging. Palm long. Fingers are almost the same size, straight but converging distally, with small gap between them. Both fingers finish in a spinous processes and have small teeth on the cutting edges, with a larger tooth appearing on the proximal third. Abdomen smooth, with rounded abdominal pleura. Telson with lateral margins converging distally to a pair of spines and then finishing in a rounded posterior margin.
Holotype measurements. Height of right cheliped 13.5 mm, thickness of right cheliped 7.2 mm, length of rigth cheliped 32.4 mm, length of carpus (5th rigth pereiopod) 6.3 mm, length of dactylus (5th right pereiopod) 3.9 mm, length of merus (5th right pereiopod) 8.8 mm, length of propodus (5th right pereiopod) 8.9mm, length of the carapace 36.7 mm, length of the abdomen 40.6 mm, length of the rostrum (to the posterior orbital edge) 5.9 mm, length of telson 12.6 mm, length of uropod 1 (endopodite) 10.1 mm, length of uropod 2 (exopodite) 12.1 mm.
Maximum size. The largest of 750 captured and released animals was 9.7 cm long (total length measured from the tip of the rostrum to end of the telson).
Colour. The colour varies according to size and substrate. Small animals are usually sand-coloured. Large animals are darker, ranging from brown to dark olive, sometimes almost purple. Fingertips of the first pereopod are red or orange in large animals.
Morphometric Comparison of P. brasiliensis promatensis and P. brasiliensis brasiliensis .
Mean residuals and residual standard deviations derived from the adjusted power regressions (y=a.Carapace_Length b) are presented at Table 4. Comparison of mean residuals (ANOVA) between P. brasiliensis promatensis and P. brasiliensis brasiliensis resulted significant for 11 different measurements.
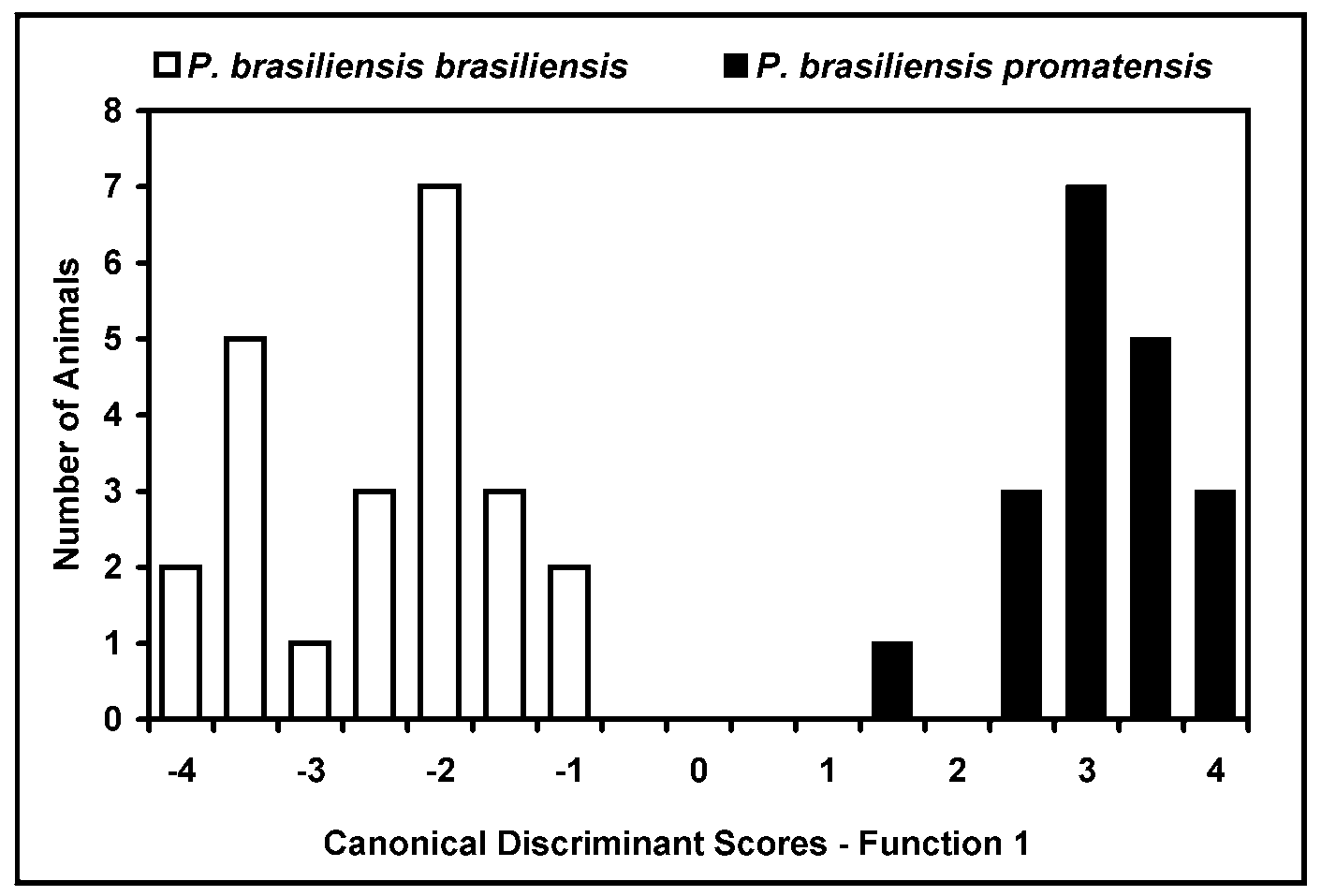
Discriminant analysis was applied to compare both species. All variance was explained by function 1 (P<0.001), with a Canonical Correlation of 0.948 and perfect (100%) membership identification. Centroid values for P. brasiliensis promatensis and P. brasiliensis brasiliensis was 3.04 and -2.78 respectively. Figure 2 View FIGURE 2 shows frequency distribution of the Discriminant scores, showing that each animal could be perfectly identified through morphometrical data. Discriminant scores are also no affected by animal size, as could be seen through Figure 3 View FIGURE 3 .
Habitat. Garapiá stream drains a small basin with a thin acid soil layer over basalt rocks, covered by grass, natural and Pinnus forests. Basin area is about 3 km 2 through an altitude of 850m before a high water fall to the coastal plains. The species was not found in lower areas of the same basin. The width of the stream ranges from 0.5 m to about 3 m, with a maximum depth of 70-80 cm. The substrate varies from fine silt to sand and small rocks. Animals are found in hollows in the stream bank during the day.
TABLE 4. Mean residuals from power regressions (y=a.Carapace_Length b) of pooled data of Parastacus brasiliensis promatensis and Parastacus brasiliensis brasiliensis . Regressions were performed using SPSS (11.0) non linear regression routine, applying Levenberg-Marquardt algorithm. Coefficients of the Canonical Discriminant function 1 are also presented (r=0.948).
P. brasiliensis promaten- P. brasiliensis brasiliensis Significance for Coefficients of the Canonical
sis comparing means Discriminant Function 1
(ANOVA)
Character Mean Std. Deviation Mean Std. Deviation
Using the identification keys provided by Buckup and Rossi (1980) and Buckup (1999), P. brasiliensis promatensis is identified as P. brasiliensis . However, Discriminant analysis (Table 4, Fig. 2 View FIGURE 2 and 3 View FIGURE 3 ) are strong enough for a clear distinction between them, with perfect (100%) membership identification.
The relationship between both subspecies is very intriguing. Between them there is a geographical (60km) and altitudinal (650m) gap with no registered occurrence of any Parastacid. Parastacus brasiliensis brasiliensis is found in the central depression of Rio Grande do Sul (Jacuí Drainage), at altitudes of no more then 200m (estimated), characterized by sandstone sediments from Triassic. By the other hand, P. brasiliensis promatensis is found 850m high (Litoral Drainage), on a basalt basin formed during the Gondwana breakdown (lower Cretaceous). Of course, both subspecies are not so old. Cretaceous geological events may be related to vicariant process related to South American parastacoid genus formation, as reported by Crandall et al. (2000). Also, no major geological event is known from Cretaceous and Pleistocene climatic changes apparently do not justify this geographical gap.
Parastacids are known to migrate outside water and to resist desiccation ( Hughes & Hillyer 2003). Some years ago, an individual of P. brasiliensis brasilienis escaped from an aquarium in our lab and was found alive some (2-3) days before. The animal was so dehydrated that when returned to the water was unable to sink. Nevertheless, confined inside a hollow under water, in just some hours the animal was behaving as a healthy individual.
Parastacids can also move large distances using constructed or natural passages under the ground. We personally known a case of a very shallow (1,2m deep) water well constructed on a hill (100-200m) in sandstone sediments. The nearest creek was at least 500m distant and 20-30m bellow. After one year, the well was opened for cleaning and there were crayfishes ( Parastacus pilimanus ) living inside.
Hughes & Hillyer (2003) analysed, using molecular data, the patterns of connectivity among populations of Cherax destructor (Parastacidae) in Western Queensland, Australia. Although this species is able to terrestrial migration, these authors found almost no sharing of haplotypes across drainage boundaries, indicating limited terrestrial dispersal across them. Similar results were obtained by Nguyen e t al. (2005).
Crayfish dispersal between drainages was reported by Horwitz & Knott (1995) as food items frequently translocated by Australian aborigines. Amerinds also use crustaceans as regular food items, although with no record of Parastacus consumption.
So, the distribution of P. brasiliensis brasiliensis and P. brasiliensis promatensis so far apart is very interesting and maybe a molecular approach will be necessary to solve this biogeographical and evolutive question.
TABLE 2. Morphometrical measurements of Parastacus brasiliensis promatensis subsp. n..
| Measurement | Average | SD | Maximum | Minimum |
|---|---|---|---|---|
| HCHE | 10.45 | 3.17 | 18.00 | 5.00 |
| TCHE | 6.05 | 1.85 | 10.80 | 2.80 |
| LCHE | 26.27 | 9.75 | 52.20 | 11.70 |
| LCAR5 | 4.86 | 1.39 | 7.90 | 2.60 |
| LDAC5 | 3.63 | 0.86 | 5.20 | 2.30 |
| LMER5 | 8.27 | 2.18 | 14.10 | 4.70 |
| LPRO5 | 8.35 | 2.17 | 12.20 | 4.60 |
| LCARAP | 33.33 | 7.65 | 46.20 | 19.60 |
| LABD | 36.15 | 7.87 | 49.00 | 20.50 |
| LROS | 5.22 | 1.01 | 6.80 | 3.10 |
| LTEL | 11.15 | 2.65 | 15.60 | 6.10 |
| LURO1 | 8.95 | 2.48 | 14.50 | 4.80 |
| LURO2 | 10.64 | 2.74 | 16.00 | 5.70 |
TABLE 3. Ratios for morphometrical identification of Parastacus brasiliensis promatensis subsp. n..
| Ratio | Average | SD | Maximum | Minimum |
|---|---|---|---|---|
| Labd/Lcarap | 1.0880 | 0.0430 | 1.2188 | 1.0310 |
| Lros/Lcarap | 0.1583 | 0.0159 | 0.2227 | 0.1432 |
| Ltel/Lcarap | 0.3340 | 0.0121 | 0.3596 | 0.3112 |
| Luro1/Ltel | 0.7984 | 0.0561 | 0.9932 | 0.7121 |
| Luro2/Ltel | 0.9510 | 0.0427 | 1.0959 | 0.9000 |
| Hche/Lche | 0.4097 | 0.0478 | 0.4710 | 0.2907 |
| Tche/Lche | 0.2382 | 0.0330 | 0.2774 | 0.1547 |
| Lpro5/Lcar5 | 0.7492 | 0.0360 | 0.8248 | 0.6667 |
| Ldac5/Lcar5 | 0.3287 | 0.0303 | 0.3868 | 0.2581 |
| Lmer5/Lpro5 | 0.9936 | 0.0684 | 1.1949 | 0.9027 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |