Acanthobothrium schalli, Vardo-Zalik, Anne M. & Campbell, Ronald A., 2011
|
publication ID |
https://doi.org/ 10.5281/zenodo.206009 |
|
DOI |
https://doi.org/10.5281/zenodo.6191982 |
|
persistent identifier |
https://treatment.plazi.org/id/0398B06F-5C6C-E177-F6E0-FB84FCFCFF34 |
|
treatment provided by |
Plazi |
|
scientific name |
Acanthobothrium schalli |
| status |
sp. nov. |
Acanthobothrium schalli sp. nov.
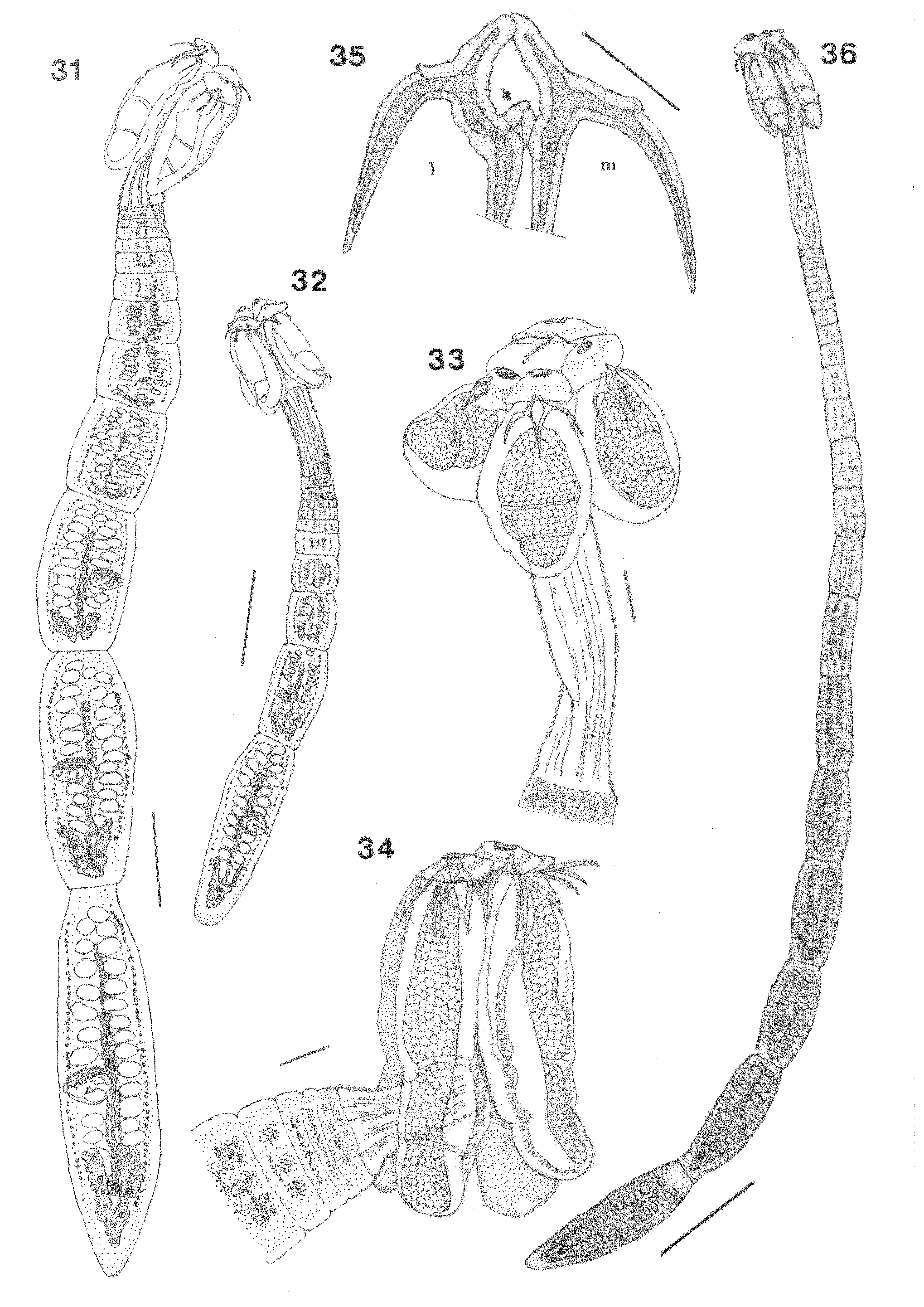
( Figs. 14–19 View FIGURES 14 – 19 , 35, 36 View FIGURES 31 – 36 )
Specimens deposited: holotype (USNPC 103820); paratypes (USNPC 103821–103826)
Type Host: Mustelus canis canis Mitchill ; Dusky smooth-hound; Charcharhiniformes:
Type locality: Gulf of Mexico, 26 24.20’N, 96 23.80’W at 45 fathoms 14. X.94, coll. R. A. Campbell.
Other hosts and localities: from Mustelus norrisi Springer ; Narrowfin smooth-hound; Charcharhiniformes: Triakidae . Gulf of Mexico stations: 28 07.44’N, 92 46.50’W at 45 fathoms, 28 17.86’N 94 10.94W at 27 fathoms, 28.15.20’N, 94 10.94’W at 27 fathoms, 28
15.20’N at 94 10.94’W at 29 fathoms, 28 56.70’N, 93 56.11W at 12 fathoms, 28 24.39’N,
92.20.29’W at 31.5 fathoms; coll. R. A. Campbell (vouchers USNPC 103827–103829).
Site of infection: spiral intestine.
Prevalence: Mustelus canis : 7/7 individuals examined; M. norrisi : 4/4 individuals examined.
Etymology: the species is named after Dr. Joseph J. Schall, parasitologist, University of Vermont, mentor of A. Vardo-Zalik.
Description: Based on measurements of 17 whole mounted specimens and 2 worms examined by SEM. Small worms, 3–7 mm (4.4, n=14) long, consisting of 10–23 (14, n=13) segments. Strobila acraspedote, euapolytic. Scolex 396–520 (433, n=17) long, maximum width 240–328 (284, n=15), composed of 4 triloculate bothridia free at posterior ends; each bothridium with apical sucker and pad, armed with pair of bifid hooks. Bothridia 388–496 (427, n=12) long by 100–170 (129, n=17) wide, rounded posteriorly, mean (BL: BW) 2.9: 1. Bothridia covered with spinitriches over proximal surfaces and divided into three loculi by thin muscular septa. Anterior loculus 200– 250 (221, n=19) long, middle loculus 50–70 (55, n=19) long, posterior loculus 70–100 (83, n=19) long; mean locular ratio (A: M: P) 1:0.25:0.38. Apical pad 100–130 (113, n=14) long by 95–110 (102, n=14) wide; accessory sucker 30–40 (36, n=15) long by 20–40 (27, n=15) wide, rims surmounted apically by bilobed lappets ( Fig. 16 View FIGURES 14 – 19 ).
Cephalic peduncle covered with spinitriches, 280 long when contracted to 1300 long extended (524, n=15), maximum width 72–104 (93, n=15) at junction with scolex; (BL: CPL) 1: 1.1 to 1: 3.
Hook dimensions: Medial and lateral hook handles and prongs almost equal in length. Lateral hook (n=22): A= 45–50 (50) B= 80–120 (95); C= 70–100 (87); D=120–170 (142); E= 120–165 (138); W= 50–70 (57, n=12). Medial hook (n=22): A’= 45–55 (50, n=16); B’= 90–125 (100, n= 22); C’= 60–120 (83, n= 20); D’= 130–175 (146); E’= 110–180 (136); W’= 50–80 (59, n=12). Distinct knoblike tubercle 13.3–15.2 long on underside of axial prong of each hook; ratio (THL: BL) 1: 2 –1: 2.6.
Strobila: Rows of microtriches over segment strobila create striated appearance. Immature segments, 8–18 (11, n=13) in number, initially wider than long, becoming longer than wide with maturity; all male and female genitalia apparent by segment number 10. Successive segments mature rapidly, sperm present in vas deferens by segments number 14–15. Mature segments 1–5 (3, n=14) in number, longer than wide, terminal segment rounded posteriorly, 410–1040 (700, n=18) long by 110–270 (199, n=18) wide. Genital pores alternating irregularly, 34–51% (42, n=18) of segment length from posterior end; each with shallow genital atrium. Cirrus sac in posterior half of segment, 70– 120 (94, n=14) long by 40–80 (50, n=14) wide, containing armed cirrus. Testes 25–29 (27, n=19) in number; preporal 9–11 (10, n=19), aporal 12–15 (14, n=19), and postporal 3–4 (3, n=19); sub—spherical, 40–60 (49, n=33) long by 20–50 (33, n=36) wide; arranged in two single layered columns extending from ovarian isthmus to near anterior extremity of segment. Vagina anterior to cirrus sac, thick walled, parallels anterior margin of cirrus sac from genital atrium then descends in midline to join oviduct posterior to ovarian isthmus. Vaginal sphincter absent. Seminal receptacle not observed. Mehlis’ gland posterior to ovarian isthmus, oval, c. 48 long by 28 wide. Ovary posterior, inverted-A shaped, bilobed in cross section with numerous pendulous lobules ( Fig. 19 View FIGURES 14 – 19 ); total length 176– 344 (295, n=9) by 120–192 (144, n=9) wide at isthmus, anterior arms symmetrical throughout most of strobila ( Figs.18, 19 View FIGURES 14 – 19 ) not reaching cirrus sac, poral lobe 150–350 (234), aporal lobe 150–350 (241). Ovary larger in terminal segments, lobes slightly asymmetrical, differing by c. 5 % of total length; poral lobe terminating near level of cirrus sac, aporal lobe extending to or anterior to posterior margin of cirrus sac ( Fig. 17 View FIGURES 14 – 19 ); lobes united posteriorly, encircling Mehlis’ gland ( Fig. 18 View FIGURES 14 – 19 ). Uterus thick walled, tubular, extends from the level of the ovarian isthmus to level of the third most anterior testes. Vitellarium follicular, consisting of 2 lateral columns, each 1–2 follicles deep, extending from level of penultimate testis anteriorly to ovarian isthmus posteriorly, interrupted by vagina and cirrus sac. Excretory ducts lateral. Eggs not observed.
Remarks: Acanthobothrium schalli is the first species described from sharks in the western Atlantic, occurring in all specimens examined of both M. c. canis (7) and M. norrisi (4) in the Gulf of Mexico. Acanthobothrium schalli can be differentiated from most category 1 species in possessing an ovary that has an inverted-A shape in dorsoventral view but is bilobed in cross-section, i.e. the posterior arms of the ovary are united posterior to the ovarian isthmus. The symmetrical anterior ovarian lobes in the majority of the segments of Acanthobothrium schalli is indicative of a category 1 species (SFFS) according to the parameters set by Ghoshroy & Caira (2001). The subtle elongation of the anterior aporal lobes by 5% of their length in the oldest segments is similar to the category 1 species A. foulki and Acanthobothrium peruviense Reyda, 2008 with ovaries that Reyda & Caira (2006) described as “essentially symmetrical”.
Five species of Acanthobothrium have been reported from sharks of the genus Mustelus : A. coronatum from M. mustelus in the Northeastern Atlantic and Mediterranean Sea ( Baer 1948; Rees & Williams 1965); Acanthobothrium karachiense Bilqees, 1980 ; Acanthobothrium mujibi Bilqees, 1980 and Acanthobothrium rubrum Bilqees, 1980 in Mustelus (=Myrillo) manazo Bleeker from the Arabian Sea, Karachi coast of Pakistan ( Bilqees 1980); and A. mathiasi in M. mustelus and M. canis from the Mediterranean Sea ( Euzet 1959). Acanthobothrium schalli can be distinguished from A. coronatum , A. karachiense , A. mujibi , and A. rubrum by lesser number of testes (25–29 vs. 81–115, 74–98, 36–41, and 60–87 respectively). Acanthobothrium schalli differs from A. mathiasi in being smaller (3–7mm vs. 10–20 mm) with shorter total hook length (70–100 vs. 155–200) and different ovarian form (inverted- A vs. H-shaped).
Compared to all species described from the western North Atlantic, A. schalli is most similar to A. lintoni (USNPC 62938) from the electric ray Narcine brasiliensis Olfers in the Gulf of Mexico in scolex dimensions, hook form and accessory sucker diameter. Unfortunately, the holotype of A. lintoni (USNPC 62938) is incomplete; it is 6mm long with 27 immature segments, all with underdeveloped reproductive organs. Segments of A. schalli mature more rapidly. The holotype of A. schalli is 7mm long with 18 fully discernable segments ( Fig. 36 View FIGURES 31 – 36 ) in which all reproductive organs can be recognized by segment number 10 and mature segments appear by number 14. Furthermore, A. schalli differs from A. lintoni in having larger anterior loculi (200–250 vs. 171–175 in USNPC 62938), greater locular ratio (A:M:P 4: 1: 1.5 vs. 3:1:1), striated strobila vs. “smooth” in A. lintoni , larger cirrus sac (70–120 x 40 –80 vs. 35– 84 x 13–74), larger ovary (176–344 vs. 19–51) and ovarian shape (inverted-A vs. Hshaped). Ghoshroy & Caira (2001) designated A. lintoni a possible mix of species fitting categories 1 (8, 9, 5).
Acanthobothrium schalli can be distinguished from other species in the western Atlantic having a strobila less than 10mm long, an average of less than 30 testes per segment, “spinose” peduncles and bothridia, and fewer than 30 segments per segment. Acanthobothrium schalli differs from A. himanturi by fewer testes (25–29 vs. 38–57) and larger anterior loculus (200–250 vs. 142–185); it differs from A. fogeli by the presence of postporal testes, as well as testis number; A. schalli can be distinguished from A. paulum by fewer aporal testes (12–15 vs. 17–34) and a smaller middle loculus (50–70 vs. 80–240); it is different from A. lineatum , by fewer postporal testes (3–4 vs. 5– 10) and ovarian shape (inverted-A shaped vs. H- shaped) and possesses smaller apical suckers (20–40 vs. 48–67) and larger apical pads (100–130 vs. 83–87) than A. marplatense . Of the species described herein A. schalli most closely resembles A. lentiginosum and can be differentiated by possessing more segments (10–23 vs. 5–7) and prevaginal testes (9–11 vs. 5–7). Among other species from the eastern Atlantic and Mediterranean Sea of similar size with less than 30 testes per segment, it differs from A. minus in the absence of a vaginal sphincter, larger size (7mm vs. 1–1.9 mm) and number of segments (up to 23 vs. 8). Acanthobothrium schalli lacks the bothridial lappets possessed by A. dujardinii and A. edwardsi ; it has fewer testes than Acanthobothrium batailloni Euzet, 1955 (25–29 vs. 37–64) but has more testes than A. quadripartitum (25–29 vs. 18) and A. tripartitum (25–29 vs. 13–16); and it differs from the various forms of Acanthobothrium filicolle Zschokke, 1888 in ovarian shape (inverted-A vs. Hshaped) and presence of lappets on the apical suckers (absent from A. filicolle ).
From the eastern Pacific, the category 1 species similar to A. schalli are: A. monski , A. atahualpai , A. minusculum , A. nicoyaense , and A. royi . Fewer segments (10–23 vs. 24–28) and more preporal testes (9–11 vs. 4–7) separate A. schalli from A. monski , while more testes (25–29 vs. 12–22) and a smaller apical sucker diameter (20–40 vs. 57–71) differentiate A. schalli from A. nicoyaense . In comparison with A. atahualpai , A. schalli is larger (3–7 vs. up to 2.1 mm), and has a smaller anterior loculus (200–250 vs. 272–310). Acanthobothrium schalli has more testes (25–29 vs. 6–10), and is a larger worm (3–7 vs. 1–2 mm) than A. minusculum and differs from A. royi in shape of the ovary (inverted-A vs. H-shaped).
In the Indo-Pacific region A. schalli differs from A. foulki , A. marymichaelorum , A. larsoni , A. saliki and A. southwelli in the absence of postovarian testes and lacks the weak horizontal band of musculature across the posterior loculi of A. asnihae and A. gnomus . It has fewer testes than A. guptai (25–29 vs. 44–45) and is distinct from A. oceanharvestae in fewer postporal testes (3–4 vs. 6–11), shorter ovarian lobes (150–300 vs. 380–711) and longer anterior loculi (200–250 vs. 160–190). Shorter suckers (30–40 vs. 45–70), more postporal testes (3–4 vs. 0–2) and larger anterior loculus (200–250 vs. 125–180) distinguish A. schalli from A. romanowi . Acanthobothrium schalli has a larger anterior loculus (200–250 vs. 153–181) and more testes (25–29 vs. 17–24) than A. saliki . Acanthobothrium schalli is further distinguished from A. southwelli by larger anterior and posterior loculi (200–250 and 70–100 vs. 160 and 60) and fewer testes (25–29 vs. 34). Acanthobothrium schalli can be distinguished from A. zainali by its larger anterior and posterior loculi (200–250 and 70–100 vs. 156–197 and 43–56); and like A. zimmeri it has a bilobed ovary in cross-section but the ovary is not H-shaped and the testes lie anterior to the ovarian isthmus instead of extending posterior to it. The testes of A. zimmeri are in a monolayer and presumably lie dorsal to the ovary ( Fyler et al. 2009) but this was not stated. Another conspicuous difference between A. schalli and A. zimmeri is the location of the genital pore (34–51% vs. 62–72% of segment length from posterior end).
Of the category 1 species recognized from the Australian region ( Fyler & Caira 2006) it differs from the small species in Australian waters as follows: A. bartonae by overall size (up to 7 mm vs. 2.1 mm) and lack of spurs on the lateral hook prongs (present in A. bartonae ); A. schalli possesses fewer testes than A. clarkae , A. laurenbrownae , A. martini , A. pearsoni and A. urolophi (25–29 vs. 45–52, 31–46, 56–60, 34–41 respectively); A. mooreae has a vaginal sphincter (absent in A. schalli ) and smaller anterior loculus compared to A. schalli (147–175 vs. 200– 250); A. schalli differs from A. odonoghuei by the absence of capilliform microtriches on the cephalic peduncle; A. schalli differs from A. rohdei and A. stevensi by lacking a vaginal sphincter; A. thomasae differs from A. schalli as it has bothridia longer than the cephalic peduncle.
One of smooth hound sharks examined in this study, M. norrisi View in CoL , proved to be a new host for Acanthobothrium . According to Froese & Pauly (2010) and Heemstra (1997), M. c. canis View in CoL is commonly found along the east coast of North America in coastal waters, while M. norrisi View in CoL is more abundant in northern Gulf of Mexico. Mustelus c. canis View in CoL has been examined for parasites as far north as New England with no reported Acanthobothrium specimens; however, A. mathiasi has been previously reported from this host in the Mediterranean Sea ( Euzet 1959). There are no previous published records of the parasite fauna of M. norrisi View in CoL .
| USNPC |
United States National Parasite Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Acanthobothrium schalli
| Vardo-Zalik, Anne M. & Campbell, Ronald A. 2011 |
Acanthobothrium batailloni
| Euzet 1955 |
Acanthobothrium filicolle
| Zschokke 1888 |