Leuceruthrus micropteri Marshall and Gilbert, 1905
|
publication ID |
https://doi.org/10.1645/22-36 |
|
DOI |
https://doi.org/10.5281/zenodo.7753984 |
|
persistent identifier |
https://treatment.plazi.org/id/039987AB-216E-FFE0-158F-4908AEA3969E |
|
treatment provided by |
Felipe |
|
scientific name |
Leuceruthrus micropteri Marshall and Gilbert, 1905 |
| status |
|
Leuceruthrus micropteri Marshall and Gilbert, 1905 View in CoL View at ENA
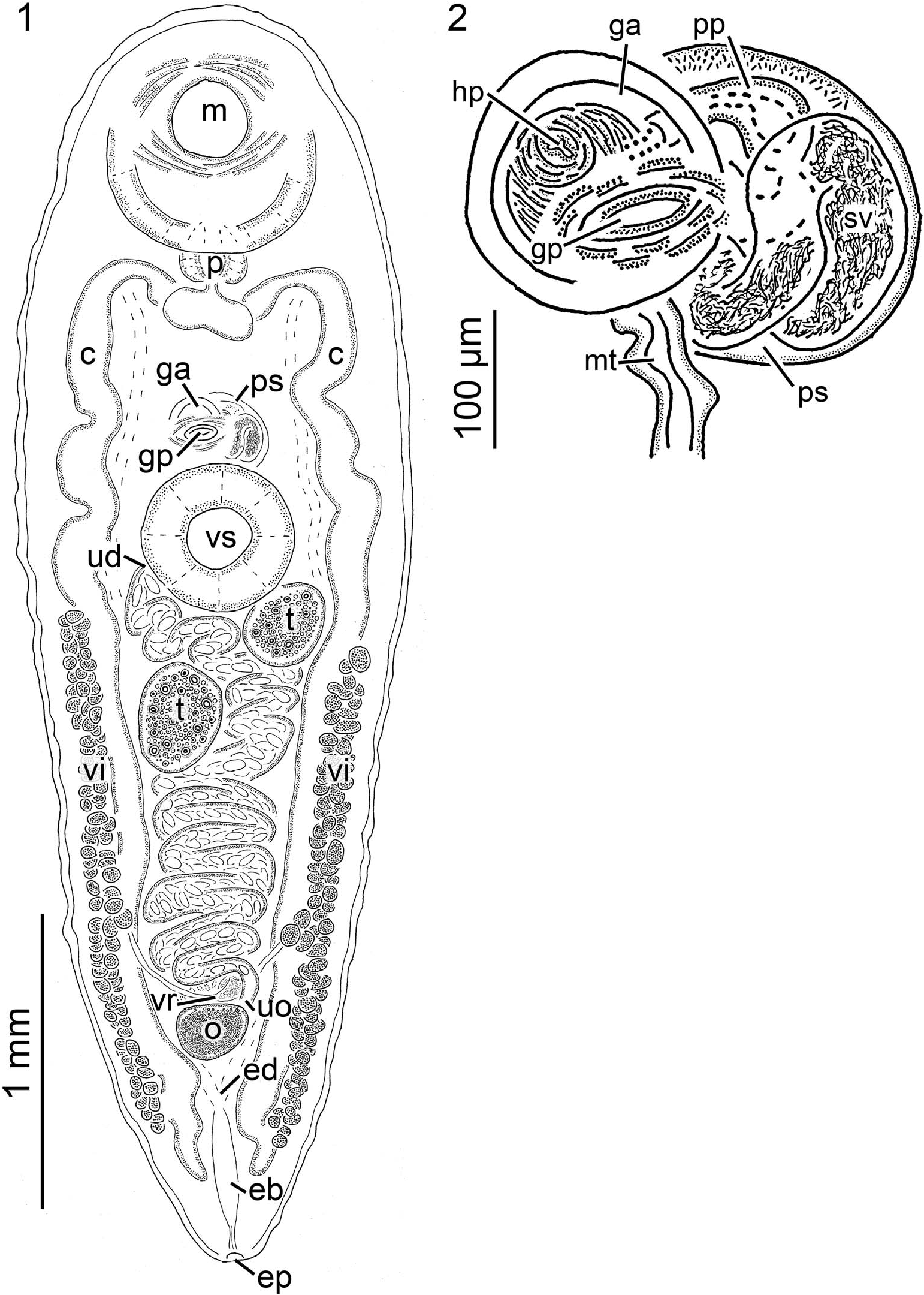
( Figs. 1, 2 View Figures 1, 2 ; Tables I View Table I , II)
Diagnosis of adult (based on light microscopy of 14 stained, wholemounted specimens) ( Figs. 1, 2 View Figures 1, 2 ): Body 3,480 5,440 (4,448, 13) [8,180] long or 2.3 3.4 (1.7, 9) [3.8] X longer than wide, width at level of oral sucker 1,280 2,060 (1,508, 12) [2,180], width at level of ventral sucker 1,240 2,060 (1,485, 12) [2,200]; forebody (¼distance from anterior end of body to the anterior most margin of ventral sucker musculature) 1,380 1,960 (1,643, 13) [2,780] long or 35 41% (37%, 13) [34%] of overall body length; hindbody (¼region of body posterior to the ventral sucker) 1,620 2,970 (2,272, 13) [4,520] long or 47 55% (51%, 13) [55%] of overall body length, 1.2 1.6 (1.4%, 13) [1.6] X greater than forebody length; tegument unarmed, smooth, approximately 20 50 (33, 13) [60] thick. Excretory system difficult to trace anteriorly, appearing Y shaped in hindbody, branches joining at 82 91% (88%, 9) [91%] of body length from anterior body end forming excretory bladder; excretory bladder 185 465 (318, 9) [690] long, 30 85 (61, 9) [230] wide, becoming confluent with diminutive excretory duct; excretory duct extending 110 195 (154, 9) [110], communicating excretory bladder and terminal excretory pore. Nervous system not evident. Oral sucker 690 910 (801, 13) [1,260] long or 17 21% (18%, 13) [15%] of body length or 43 54% (49%, 13) [45%] of forebody length, 685 920 (814, 13) [1,280] wide or 44 60% (54%, 13) [59%] of body width, anterior margin 2 5% (3%, 13) [3%] of body length from anterior body end, posterior margin 520 850 (690, 13) [1,280] from anterior margin of ventral sucker ( Fig. 1 View Figures 1, 2 ). Ventral sucker in anterior half of body, 480 600 (533, 13) [880] long or 10 14% (12%, 13) [11%] of body length, 470 670 (573, 13) [820] wide or 33 45% (38%, 13) [38%] of body width, 62 75% (67%, 13) [69%] of oral sucker length 65 74% (70%, 13) [64%] of oral sucker width ( Fig. 1 View Figures 1, 2 ). Mouth opening ventrally. Pharynx ovoid, 185 280 (232, 13) [370] long or 4 6% (5%, 13) [4%] of body length 215 285 (251, 13) [400] wide ( Fig. 1 View Figures 1, 2 ). Esophagus extending posteriad from mouth approximately 175 435 (341, 13) [310] before bifurcating 25 70 (40, 13) [40] posterior to pharynx; dextral esophageal branch 130 255 (172, 13) [230] long, sinistral esophageal branch 110 215 (164, 13) [210] long ( Fig. 1 View Figures 1, 2 ); dextral cecum 2,555 4,090 (3,555, 13) [7,020] long or 69 93% (80%, 13) [86%] of body length, prececal space, 820–1,140 (949, 13) [1,500] or 19 24% (21%, 13) [18%] of body length from anterior end of body, postcecal space,100 360 (239, 13) [240] or 2 7% (5%, 13) [3%] of body length from posterior end of body; sinistral cecum 2,525 4,035 (3,535, 13) [7,100] long or 70 93% (80%, 13) [87%] of body length, prececal space 820–1,140 (954, 13) [1,500] or 19 24% (22%, 13) [18%] of body length from anterior end of body, postcecal space 100 420 (264, 13) [400] or 2 8% (6%, 13) [5%] of body length from posterior end of body.
Testes oblique to askew, suboval, typically asymmetrical ( Fig. 1 View Figures 1, 2 ); dextral testis 220 425 (333, 13) [550] long or 5 10% (8%, 13) [7%] of body length, 215 425 (282, 13) [600] wide or 14 22% (19%, 13) [28%] of body width, pretestis space 1,740 2,820 (2,194, 13) [4,220] from anterior end of body or 42 59% (50%, 13) [51%] of total body length, posttestis space 1,395 2,780 (1,927, 13) [3,410] from posterior end of body or 33 51% (43%, 13) [42%] of total body length; sinistral testis 250 405 (334, 13) [500] long or 6 10% (8%, 13) [6%] of body length, 220 415 (288, 13) [470] wide or 14 26% (19%, 13) [22%] of body width, pretestis space 1,840 2,680 (2,186, 13) [3,520] from anterior end of body or 43 56% (49%, 13) [51%] of total body length, posttestis space 1,260 2,520 (1,925, 13) [4,160] from posterior end of body or 36 50% (43%, 13) [43%] of total body length. Vasa efferentia indistinct in whole-mounted specimens. Prostatic sac anterior margin 295 590 (448, 13) [890] from posterior margin of oral sucker, 220 370 (275, 13) [450] long, 200 295 (245, 13) [300] wide ( Fig. 1 View Figures 1, 2 ). Seminal vesicle thin walled, highly convoluted, filled with sperm, swollen for entire length, occupying most of prostatic sac, 395 940 (695, 13) [1,065] long ( Fig. 2 View Figures 1, 2 ), distal end connected to pars prostatica via verschlussapparat ( Womble et al., 2015). Pars prostatica diminutive, spherical, 125 185 (163, 10) [215] long, 40 65 (51, 9) [85] wide proximally, 20 40 (27, 9) [40] wide distally, exiting prostatic sac ventrally ( Fig. 2 View Figures 1, 2 ). Ejaculatory duct (¼continuation of pars prostatica outside of prostatic sac) straight, extending ventrally from prostatic sac becoming confluent with hermaphroditic duct, 40 105 (63, 6) long or 23 60% (39%, 6) of pars prostatica length. Sinus organ difficult to trace in ventral orientation, directed ventrally. Hermaphroditic pore level with midline of prostatic sac, anterior of ventral sucker, at 32 37% (34%, 11) [31%] of body length from anterior end of body ( Fig. 2 View Figures 1, 2 ). Genital atrium circular in outline, 130 260 (186, 12) [415] in diameter, 4 of 13 (33%) specimens containing 4 5 (4.3, 4) uterine eggs ( Fig. 2 View Figures 1, 2 ). Genital pore opening ventrally at 32 41% (35%, 13) [32%] of body length from anterior end ( Figs. 1, 2 View Figures 1, 2 ).
Ovary 105 315 (200, 13) [325] long or 2 7% (5%, 13) [4%] of body length, 180 375 (275, 13) [420] wide or 13 23% (18%, 13) [19%] of body width, postovary space 600 1,070 (764, 13) [1,240] or 13 21% (17%, 9) [15%] of body length ( Fig. 1 View Figures 1, 2 ). Fine details of the female genitalia (i.e., oviduct, Laurer’s canal, ovovitelline duct, and oötype) not evident, putative highly glandular Mehlis’ complex present immediately anterior to ovary. Uterus comprising a field 1,040 2,000 (1,718, 12) [4,060] long or 30 45% (39%, 12) [50%] of body length, proximal end briefly extending anteriad from ovary and Mehlis’ complex, looping laterally between ceca posterior to testes, becoming narrow and extending between testes, passing dorsal to musculature of ventral sucker, synthesis with metraterm distally near prostatic sac, typically with hundreds of eggs ( Fig. 1 View Figures 1, 2 ); uterine seminal receptacle present, detected in observations of live specimens; metraterm thick walled, 200 495 (331, 10) [340] or 4 12% (8%, 9) [4%] of body length, 35 60 (51, 9) [55] wide, extending anteriad from commissure with uterus, becoming confluent with distal portion of ejaculatory duct, forming a short common duct (¼herein a ‘‘hermaphroditic duct’’) within sinus organ ( Fig. 2 View Figures 1, 2 ). Vitellarium extending from near posterior margin of ventral sucker to near posterior end of body ( Fig. 1 View Figures 1, 2 ), maximum distance between fields 720 1,280 (889,13) [1,360] or 54 66% (59%, 13) [62%] of body width; dextral vitelline field 1,160 2,240 (1,788, 13) [3,440] long or 33 50% (40%, 13) [42%] of body length, terminating anteriorly at 46 57% (50%, 13) [48%] of body length, terminating posteriorly at 87 97% (91%, 13) [90%] of body length, 43 62% (50%, 13) [49%] of dextral cecum length; sinistral vitelline field 1,180 2,380 (1,756, 13) [3,480] long or 34 48% (39%, 13) [43%] of body length, terminating anteriorly at 46 56% (51%, 13) [53%] of body length, terminating posteriorly at 89 95% (91%, 9) [94%] of body length, 40 62% (50%, 13) [49%] of sinistral cecum length; primary vitelline collecting ducts nearly symmetrical, extending posteromediad from respective vitelline field before becoming confluent and forming vitelline reservoir; dextral vitelline collecting duct 325 605 (470, 8) [840] long, 15 60 (30, 8) [30] wide near yolk reservoir, proximal end branches from vitellarium at 43 64% (53%, 8) [50%] of dextral vitelline field length; sinistral vitelline collecting duct 275 550 (439, 8) [1,100] long, 20 35 (27, 8) [50] wide near yolk reservoir, proximal end branches from vitellarium at 44 59% (53%, 8) [66%] of sinistral vitelline field length. Vitelline reservoir near posterior margin of ovary ( Fig. 1 View Figures 1, 2 ). Eggs pyriform, varying in size from approximately 50 65 (59, 8) [50] X 25 35 (29, 8) [25] to approximately 70 95 (79, 8) [95] X 35 45 (39, 8) [40].
Taxonomic summary for Leuceruthrus micropteri
Type host: Micropterus salmoides (Lacepe`de, 1802) (Centrarchidae) , largemouth bass.
Other hosts: Tables I View Table I , II.
Type locality: Lake Mendota, Lake Monona, and Lake Wingra ( Wisconsin) .
Other localities: Tables I View Table I , II.
Specimens examined: 1 whole-mounted adult specimen (cotype, USNM 51685) and a series of slides comprising sections of an adult specimen (USNM 10678).
Specimens and sequences deposited: Vouchers (USNM 1593581, 1593582, 1593583); ITS2 sequences (GenBank ON87234, ON877235 View Materials ).
Site in host: Adults infecting stomach.
Prevalence and intensity of infection: 14 of 17 (82%) largemouth bass were infected by 1–5 (mean intensity ¼ 3) adult specimens of L. micropteri .
Taxonomic remarks on Leuceruthrus micropteri
We could not differentiate our adult specimens of L. micropteri from the cotype (a whole-mounted adult specimen, USNM 51685) so we considered our adult specimens from Wheeler Reservoir largemouth bass to be conspecific with the Marshall and Gilbert (1905) specimens of L. micropteri (see Marshall and Gilbert, 1905; Goldberger, 1911). However, we retain some uncertainty about the identity of L. micropteri , because no recent collection from the type locality has described adults and cercariae and no sequence data exist from the type locality. Future workers should sample and examine pleurocerids (Table II) and centrarchids (the type host is reportedly ‘‘largemouth bass’’) from the type locality (Lake Mendota, Lake Monona, and Lake Wingra) (these lakes are part of the Rock River, a tributary of the Mississippi River) ( Tables I View Table I , II). Simply, it could be that the specimens we call L. micropteri herein are slightly morphologically or genetically distinct from the species that Marshall and Gilbert (1905) and Goldberger (1911) collected, but these morphological differences are likely within the cercaria; which were not described by Marshall and Gilbert (1905) nor Goldberger (1911). Adults and free-swimming cercariae of L. micropteri from the type locality would be a nice museum contribution, because the cotype (an adult) is in poor condition and the only other type materials comprise some sections of an adult. An important study in North Elkhorn Creek, Kentucky ( Ohio River), by Rosen et al. (2019) used ITS2 sequences to match a cercaria they collected from fine-ridged elimia, Pleurocera semicarinata (Say, 1829) to our sequence from an adult L. micropteri (present study) from a largemouth bass in Wheeler Reservoir, Alabama ( Tennessee River). This result indicates that at least our specimens are conspecific with those of Rosen et al. (2019). However, the fact remains that the type locality needs to be sampled. Noteworthy is that Rosen et al. (2019) is the only recent study that has imaged cercariae of L. micropteri .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Digenea |
|
Order |
|
|
Family |
|
|
Genus |