Dilophotes Waterhouse, 1879
|
publication ID |
https://doi.org/ 10.5281/zenodo.155977 |
|
DOI |
https://doi.org/10.5281/zenodo.6278068 |
|
persistent identifier |
https://treatment.plazi.org/id/039987C6-6546-FF9F-7431-C28AFB3AF992 |
|
treatment provided by |
Plazi |
|
scientific name |
Dilophotes Waterhouse, 1879 |
| status |
|
Dilophotes Waterhouse, 1879: 75 .
Type species: Dilophotes exilis Waterhouse 1878: 116 (by original designation). Stenolycus Ohbayashi, 1956: 58, syn. n.
Type species: Stenolycus ohirai Ohbayashi, 1956: 58 (by original designation).
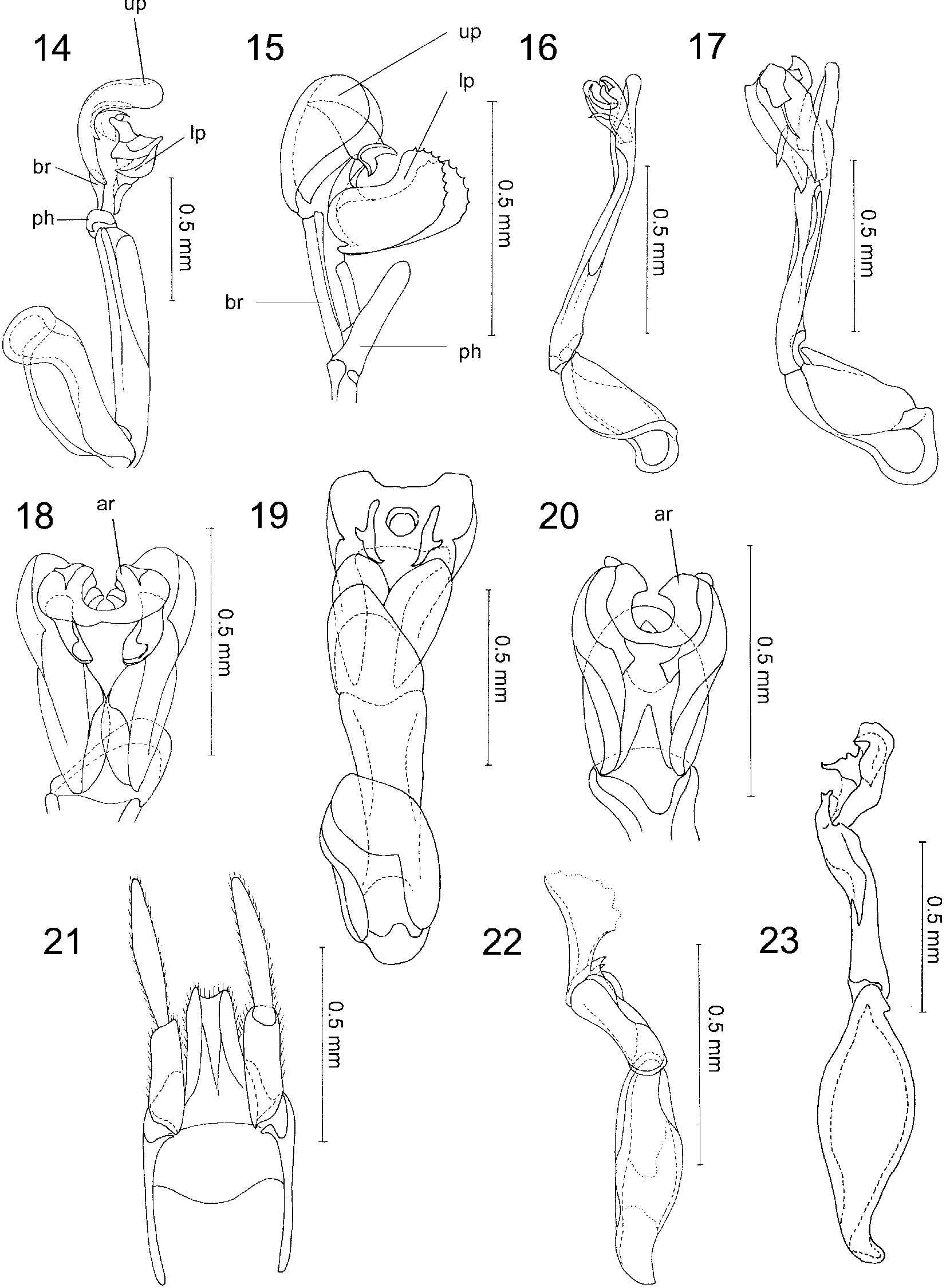
Diagnosis. Dilophotes , together with Macrolycus Waterhouse, 1878 , forms the tribe Macrolycini ( Lycinae ). Macrolycini are easily recognised by cleft claws and the structure of pronotal carinae ( Figs 6 8 View FIGURES 3 13 ). These genera differ externally in the number of longitudinal elytral costae. Dilophotes has costa 1 considerably shorter than costa 2 and 4, costa 3 is vestigial and distinct only at the very base of elytra. Costae 2 and 4 reach the apical part of elytra ( Fig. 3 View FIGURES 3 13 ). Macrolycus has four longitudinal costae on each elytron, although some of them may be very weak, especially apically. Dilophotes often have a considerably sclerotized and exposed internal sacs, which sometimes form a very complex structure ( Figs 12 View FIGURES 3 13 , 15, 18, 20 View FIGURES 14 23 ). In addition, the phallobase of Dilophotes is long and asymmetrical ( Figs 14, 16, 22 View FIGURES 14 23 ). Macrolycus has a simple, slender, membranous internal sac and at most its apical part is exposed; its phallobase is small and rounded Bocak and Bocakova (1990).
Redescription. Body small to medium sized, length 5.1 13.2 mm, mostly 6.0 8.0 mm, parallelsided to slightly widened posteriorly, moderately dorsoventrally depressed, weakly sclerotized. Many species brightly coloured, with dark red to orange yellow pronotum and elytra. Only few species concolorous black. Head hypognathous, small, slightly transverse, with short, conical rostrum. Eyes small, almost regularly smaller than their frontal interocular distance, hemispherically prominent, seldom with maximum eye diameter considerably larger than interocular distance. Frons concave, with deep depression medially, antennal tubercles flat, but distinct. Clypeus widely concave, labrum transverse, emarginate apically. Antennae slender, compressed, reaching to two thirds the length of elytra, either uniform in sexes or male antennae flabellate and female antennae weakly serrate. Flabellae of antennomeres 3 10 up to four times longer than trunks of antennomeres. Mandibles elongate, slender, curved, their inner margins simple. Maxillae with long setose galea. Maxillary palpi 4segmented, basal palpomere very small, triangular, palpomere 2 almost three times longer than basal one, palpomere 3 shorter, apical palpomere robust, as long as wide, securiform or parallelsided. Labial palpi 3segmented, considerably shorter, basal palpomeres minute, their combined length slightly longer than those of apical palpomeres, which resembles in shape those of maxillary palpi. Pronotum flat, with only short longitudinal pronotal keel present anteriorly, sometimes with a transverse mound present at middle of pronotum. Frontal margin usually widely rounded, frontal angles inconspicuous, lateral margins straight or slightly concave, posterior angles projected, sometimes very acutely. Disc rugose at frontal and lateral margins, shiny in middle and at base. Scutellum small, simply rounded at posterior margin. Elytra flat, slightly sclerotized, with 3 distinct longitudinal costae in anterior half of elytra. Costa 1 regularly shortened, seldom reaching beyond half of elytra, costae 2 and 4 complete, running entire length of elytra, costa 3 vestigial, scarcely distinct at base of elytra. Transverse costae absent, interstices finely rugose, densely pubescent. Legs slender, compressed, claws cleft apically. Male genitalia consisting of phallobase, phallus, and internal sac ( Fig. 14 View FIGURES 14 23 ). Parameres absent. Phallobase long, slender, asymmetrical, phallus mostly slender, simple. Internal sac often completely sclerotized and in some cases consisting of a pair of basal rods and lower and upper plate ( Figs 12 View FIGURES 3 13 , 15 View FIGURES 14 23 ). Female genitalia are characterized by elongate styli and relatively short coxites. Valvifers fused with coxites and bases of coxites connected by bridge ( Figs 10 View FIGURES 3 13 , 21 View FIGURES 14 23 , 24 View FIGURES 24 26 ).
Sexual dimorphism. There is only very slight sexual dimorphism in body shape and size. Males and females differ only in several species in the shape of antennae. Dilophotes ohirai ( Ohbayashi, 1956) , D. vandykei ( Nakane, 1970) , D. luteus sp. n., D. anthracinus sp. n., and D. lizipingensis sp. n. have flabellate antennae in males ( Fig. 4 View FIGURES 3 13 ) and very slightly serrate antennae in females ( Fig. 5 View FIGURES 3 13 ). Most species have relatively small eyes without any substantial sexual dimorphism. Only males of D. luteus sp. n. and D. kubani sp. n. have very large eyes. Unlike many other genera, Dilophotes females have large eyes similar to those of the males.
Remarks. Waterhouse (1878) presented a description of the genus without proposing its formal name. The paragraph was headed "Genus 38" and the single included species was Lycus exilis . Waterhouse (1879) then named this genus Dilophotes and included two species in it: Dilophotes exilis (Waterhouse, 1978) and D. pygmaeus Waterhouse, 1879 . In this valid description he designated D. exilis as the type species of the genus.
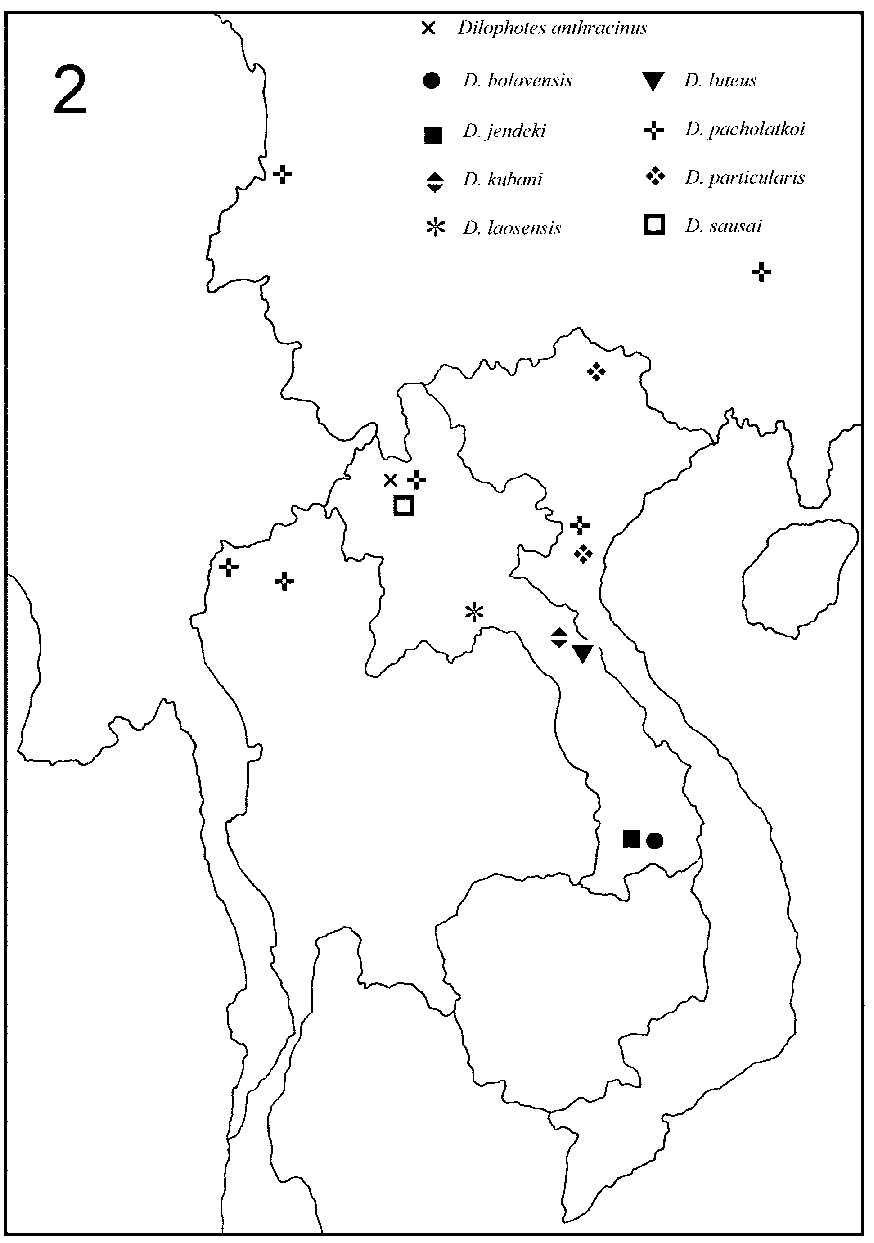
Distribution. Dilophotes occurs in the whole Oriental Region and in he eastern part of the Palaearctic Region. Unlike its relative Macrolycus , its range does not reach into Northern China or the Russian Far East. This revision shows that a very high diversity of Dilophotes exists in the tropical forests of northern Laos and it indicates the restricted ranges of most known species. The distribution of species is shown in Figs 12 View FIGURES 1 2 .
Ecology. The Chinese Dilophotes species were collected from lower mountain forests up to the high mountain forests in Northern Sichuan and Southern Shaanxi. Similarly, the species from Bhutan were collected in high mountains. Adults were slow moving or sitting on leaves of shrubs and trees in clearings in montane forests. Therefore most specimens were collected by sweeping or as individuals. The Chinese species D. atrorufus was collected in numbers on flowering Castanopsis . Larvae of Dilophotes are unknown, but development in rotting wood is expected as in the related genus Macrolycus .
The Indochinese species were collected in habitats from lowland to lower mountains and they were often collected in the lower strata of dense forest canopy (L. Bocak, personal communication).
Lycids are protected from predators by the presence of several odiforous and bitter compounds ( Moore & Brown, 1981), and therefore have a very strong tendency to form mimicry complexes. I have found that the Palaearctic species are dark red to reddish brown, similar to the other lycids that occur in the same habitat ( Macrolycus , Calochromus GuérinMéneville, 1833 and Plateros Bourgeois, 1879 ). The Bhutanese species Dilophotes bhutanensis sp. n. is similar in the body size, shape and colouration the sympatrically occurring Macrolycus bowringi Waterhouse, 1878 , unlike the second sympatrical species, D. holzschuhi sp. n. which is similar to the much smaller, dark red coloured Plateros species. Similarly, I found that in Laos's fauna the lightly coloured species are similar to some Cautires Waterhouse, 1879 and Xylobanus Waterhouse, 1879 as well as the dark red species of some Plateros and Conderis Waterhouse, 1879 .
Species level relationship. Dilophotes species are, in many cases, hardly distinguishable when comparing external characters. The body colouration is usually the same in several sympatrically occurring species. I found the principal variation in the shape of male genitalia, the shape of antennae ( Figs 4, 5 View FIGURES 3 13 ), and the relative size of the eyes. Unfortunately, only few specimens were collected in copula or under conditions where I could associate males and females of the respective species. Therefore, the characters based on female genitalia were not been used for the study of species level relationships. Based on the structure of the male genitalia I have distinguished several species groups. The shape of phallus and the degree of sclerotization and the shape of the internal sac are very diversified in Dilophotes . Unfortunately, the only related genus, Macrolycus , has a very different type of phallus and therefore I was not able to homologize the characters found in the male genitalia.
The eyes are almost regularly considerably smaller than their interocular distances. Only two species, D. luteus sp. n. and D. kubani sp. n. have very large eyes. These species differ in both the shape of their antennae and the type of their male genitalia. Therefore, the size of eyes is not indicative of their relationships.
Two types of male antennae were found in Dilophotes . Dilophotes ohirai ( Ohbayashi, 1956) , D. vandykei ( Nakane, 1970) , D. luteus sp. n., D. anthracinus sp. n. and D. lizipingensis sp. n. have flabellate antennae in males, the remaining species have very similar slender filiform to serrate antennae in both sexes. Until now, all the species with flabellate antennae were placed in Flabellodilophotes Pic, 1912 (Dilophotellus Kleine, 1925) or Stenolycus Ohbayashi, 1956. I have found that the presence and/or lack of lamellae on antennae did not correlate with the shape of the male genitalia. Flabellodilophotes is a polyphyletic, typologically based genus and can not be accepted in the classification. I examined some Oriental representatives of Flabellodilophotes and both serrate and flabellate male antennae were found for example in the D. moxiensis group, which is defined in this article (e. g. D. costatus Kleine, 1930 ). As I did not study the type species of Flabellodilophotes , I do not formally synonymize it with Dilophotes . On the other hand, species with flabellate antennae from the studied area are here classified in Dilophotes .
Nakane (1969) redescribed the Japanese species Stenolycus ohirai in detail, which is a type species of Stenolycus. I compared the male genitalia with those of species occurring in continental Asia and I have not found any principal differences between them. Stenolycus ohirai belongs to the Dilophotes anthracinus group and I propose Stenolycus to be a junior synonym of Dilophotes .
Kasantsev (2000) described a new subgenus Biphylodes as a Dilophotes without elytral costa 1. The male genitalia of the type species are similar to those of D. moxiensis sp. n. and D. pacholatkoi sp. n. These species have well developed costa 1 as all other Dilophotes . Therefore, Biphilodes as a subgenus is ill based and refers only to one species within D. moxiensis group as defined further. Additionally, some species now classified by Kleine and Pic in Flabellodilophotes Pic, 1912 have the same type of aedeagus and when the male of the Flabellodilophotes species is found, Biphilodes Kasantsev, 2000 can be a junior synonym of Flabellodilophotes Pic, 1912 or Dilophotellus Kleine, 1925.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Dilophotes Waterhouse, 1879
| Bic, Vlastislav 2002 |
Dilophotes
| Waterhouse 1879: 75 |