Lepidoptera
|
publication ID |
https://doi.org/ 10.5281/zenodo.274044 |
|
DOI |
https://doi.org/10.5281/zenodo.5613515 |
|
persistent identifier |
https://treatment.plazi.org/id/039C87C3-FFCF-DC20-FF3E-E367FC0EFAD2 |
|
treatment provided by |
Plazi |
|
scientific name |
Lepidoptera |
| status |
|
Lepidoptera View in CoL monophyly and affinities
The monophyly of the 'order' Lepidoptera is firmly established by an impressive suite of synapomorphies of its constituent basal lineages. The position of the group within the insect hierarchy is similarly well established: It has a strongly supported sistergroup relationship to the Trichoptera (caddisflies), constituting with the latter the high-rank taxon Amphiesmenoptera. The Amphiesmenoptera, in turn, together with the (similarly monophyletic?) Antliophora (Mecoptera s.lat. [including the Nannomecoptera, Neomecoptera and Siphonaptera] + Diptera), constitute the Mecopterida or 'panorpoid' clade within the Endopterygota; for supraordinal relationships see, e.g., Kristensen (1999), Beutel & Pohl (2006).
A detailed reconstruction of the lepidopteran ground plan was presented by Kristensen (1984) and updated in Kristensen & Skalski 1998). Several apomorphies arguably unique to the Lepidoptera were identified, and another set of groundplan traits was identified as probable synapomorphies with the Trichoptera , i.e., autapomorphies of the Amphiesmenoptera. The great majority of the characters in question occurs in the adult insect. Subsequent work has suggested little in the way of change.
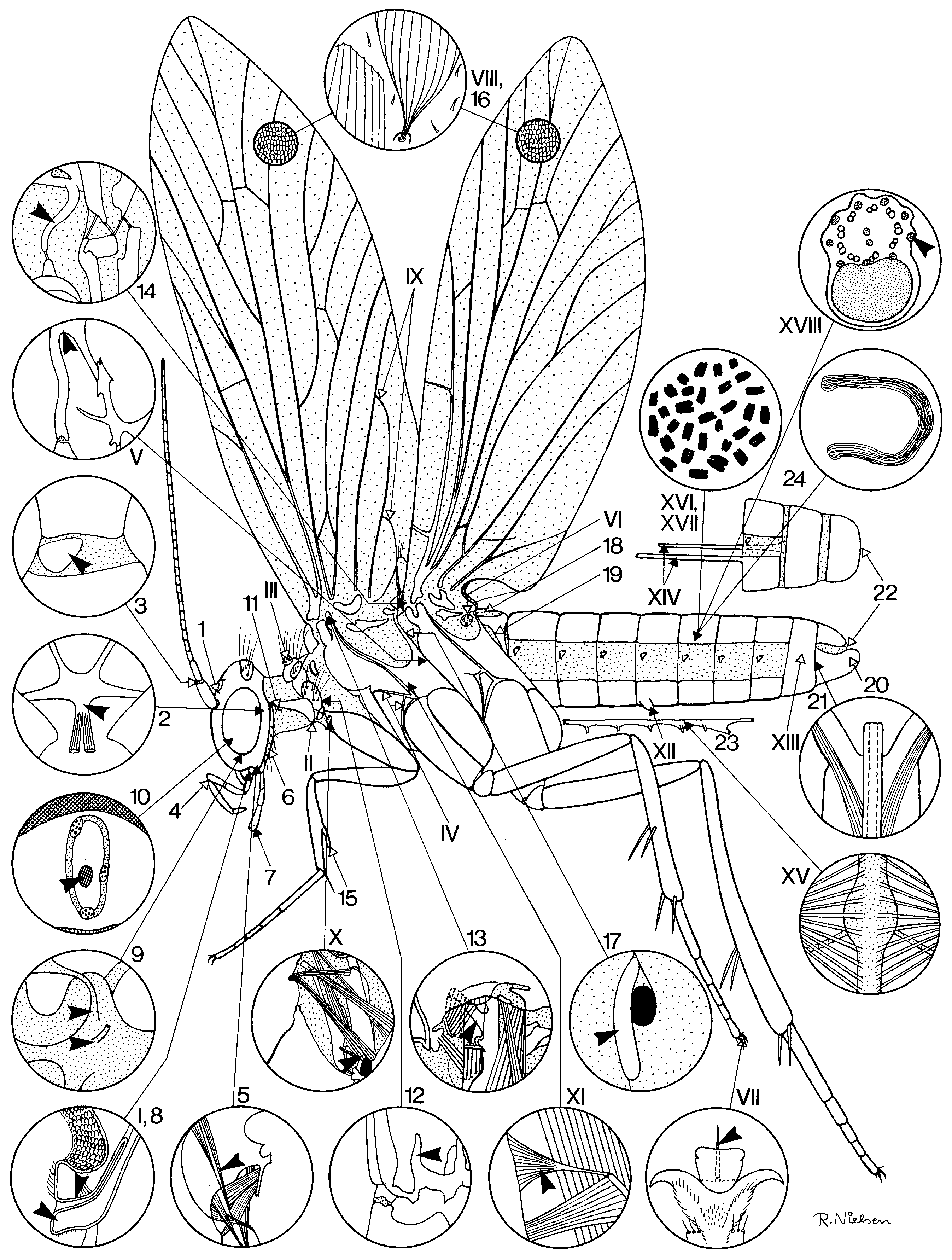
The following morphological character states are currently considered likely lepidopteran groundplan autapomorphies: Adult (numbers in brackets refer to figured details in Fig.1 View FIGURE 1 ): Median ocellus lost (1). Corporotentorium with posteromedian process, accommodating insertions of ventral neck muscles (2). Intercalary sclerite present laterally in membrane between antennal scapus and pedicellus (3). Maxillary palp with points of flexion between segments 1/2 and 3/4; segment 4 longest; intrinsic palp musculature not comprising antagonistic pairs (4). Craniostipital muscle present, slender and running close to craniocardinal muscle (5). Postlabium an arched sclerite with long piliform scales (6). Terminal segment of labial palp with group of sensilla in depression ('vom Rath's organ') (7). Labral nerve and frontal ganglion connective separating immediately at their origin on the tritocerebrum (9). Nervus recurrens running inside aorta until reaching retrocerebral complex (10). Laterocervical sclerite with proprioceptive 'hair plate' close to anterior apex (11). Prothoracic endoskeleton with prominent free arm arising from bridge between sternum and lower posterior corner of pleuron (12). Mesothorax with 'tergopleural apodeme' issued from upper part of pleural suture and accommodating insertion of a tergopleural muscle (13). Metathorax with 'prescutal arm' (14). Fore tibia with movable 'epiphysis' on inner surface, and with at most a single spur (15). Wings with dense covering of broad scales (16). Metathoracic spiracle with single, anteriorly situated, external lip (17). Tergum I extensively desclerotized, with concomitant loss of external layer of 'short' dorsolongitudinal I/II muscles (18). Tergum I with lateral lobes extending posteroventral to articulate with anterior corners of sternum II (19). Male 'valve' (gonopod) primarily undivided (20). Phallic protractor muscle originating inside gonopod (‘valve’) (21). Cerci lacking in both sexes (22). Abdominal nerve cord with at most five ganglionic masses, and unpaired connectives (23). Apyrene sperm present (24). Larva: Pleurostome elongated, craniocardinal articulation far behind mandibular base. Maxillary palp with no more than 3 segments.
Absence of a dorsolongitudinal muscle on the adult's salivarium (8) has been considered an additional lepidopteran autapomorphy, but unpublished observations on nannomecopterans (R. Beutel, pers. comm.) and trichopterans render it uncertain that a presence of a formation of this kind is actually the groundplan condition in the Mecopterida.
In the light of this robust morphological support for the monophyly of the Lepidoptera , inclusive of the Micropterigoidea, the assignment ( Chapman 1917, Hinton 1946, 1958) of the latter to a separate order Zeugloptera is now of historical interest only. Lepidoptera monophyly has also been supported consistently in available molecular analyses with relevant taxon sampling.
The following lepidopteran groundplan traits are apparently autapomorphic of the superorder Amphiesmenoptera. Adult (Roman numerals are details in Fig.1 View FIGURE 1 ): Prelabium fused with hypopharynx (I). Lower posterior corner of laterocervicale produced towards the prosternum (II). Pronotum with paired setose 'warts' (III). Prothoracic episterna with unique suture pattern (IV). Secondary furcal arms of pterothorax fused with posterior margins of corresponding epimera (V). Metathorax with setose, presumably proprioceptive, sclerite in wing base membrane behind/below subalare (VI). Pretarsus above claw with 'pseudempodium' (strong seta on socket) (VII). Wings with dense vestiture of setae (forerunners of the lepidopteran scales)(VIII). Fore wing anal veins looping up into double-Y formation (IX). One ventral (tentorial) neck muscle originating on fore coxa (X). Conical furcopleural muscle in mesothorax with broad end on pleural ridge (XI). Paired glands opening on sternum V (XII). Male segment IX with tergum and sternum fused into closed ring (XIII). Anterior margin of female segments VIII and IX with long rod-like apodemes accommodating insertions of protractor and retractor muscles of extensible oviscapt (XIV). Note: recent work shows that the interpretation of the female postabdomen in the lowest Amphiesmenoptera is more problematical than hitherto believed, and the apodemes in question may not all be homologous ( Kristensen 2003); a particularly intriguing question is whether a three-apophysis-pair configuration (with both dorsal and ventral apophyses originating on VIII) could prove ancestral in Amphiesmenoptera, since three pairs are present in Agathiphaga as well as in the enigmatic recently described caddisfly Fansipangana ( Mey 1996) . Ventral diaphragm muscles inserting on the nerve cord (XV). Female sex heterogametic (XVI). Chromosome number unusually high (basic number 30–31), chromosomes holocentric and oogenesis achiasmatic (XVII). Spermatozoa with outer accessory filaments thickened, filled with proteinaceous and glycogen-like material (XVIII). Larva: stemmata each with one crystalline cone cell transformed into primary pigment cell, hence in transverse section the cone is seen to be only tripartite. Prelabium and hypopharynx fused into composite lobe with silk gland orifice on apex.
Evidence suggests that larvae of members of the amphiesmenopteran stem lineage were 'soil animals', living in wet conditions, like those of most extant Lepidoptera-Micropterigidae. The step from here to a truly aquatic lifestyle, which is autapotypic of immature Trichoptera , is but a small one. There is every reason to believe that these larvae were free-living, for the use of silk as part of pre-pupating larval behaviour first evolved, as far we know, in the stem lineage of the Neolepidoptera.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.