Opisthjapyx naledi Sendra & Sánchez-García, 2023
|
publication ID |
https://doi.org/ 10.5852/ejt.2023.894.2287 |
|
publication LSID |
lsid:zoobank.org:pub:11C1DFE4-02F2-4FEA-BAD1-ACCAEA3590DB |
|
DOI |
https://doi.org/10.5281/zenodo.8389011 |
|
persistent identifier |
https://treatment.plazi.org/id/84437061-F133-4C0E-AF62-73FF29ADCAA1 |
|
taxon LSID |
lsid:zoobank.org:act:84437061-F133-4C0E-AF62-73FF29ADCAA1 |
|
treatment provided by |
Plazi |
|
scientific name |
Opisthjapyx naledi Sendra & Sánchez-García |
| status |
sp. nov. |
Opisthjapyx naledi Sendra & Sánchez-García sp. nov.
urn:lsid:zoobank.org:act:84437061-F133-4C0E-AF62-73FF29ADCAA1
Figs 16–23 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Etymology
The epithet naledi refers to Homo naledi , an extinct hominid species discovered within the Dinaledi chamber located in the Rising Star Cave, a World Heritage Site in South Africa. The cave where the japygid was found is located on the same hill as Rising Star Cave and separated approximately 425 meters.
Type material
Holotype SOUTH AFRICA • ♀; Gauteng Province, Maquassi Hills Municipality, Villa Louisa Cave ; 26º01′25.5″ S, 27º42′43.0″ E; 9 Oct. 2019: Rodrigo Lopes Ferreira leg.; labelled “♀-holotype, SAM-ENW-C015127 ”; ISAM. GoogleMaps
Description
BODY. Elongate, length 27.5 mm, maximum width at urotergite VII 2.6 mm. Epicuticle smooth under optical microscope; with few micropores visible at higher magnifications (dorsal side of urite X with 1 micropore per 10 μm 2, diameter 0.6‒0.7 μm) ( Fig. 21B View Fig ). Cuticle unpigmented with slightly sclerotized areas on dorsal frontal head, mandible tips, femoral and tibial condyles, abdominal segments VIII−X, and cerci.
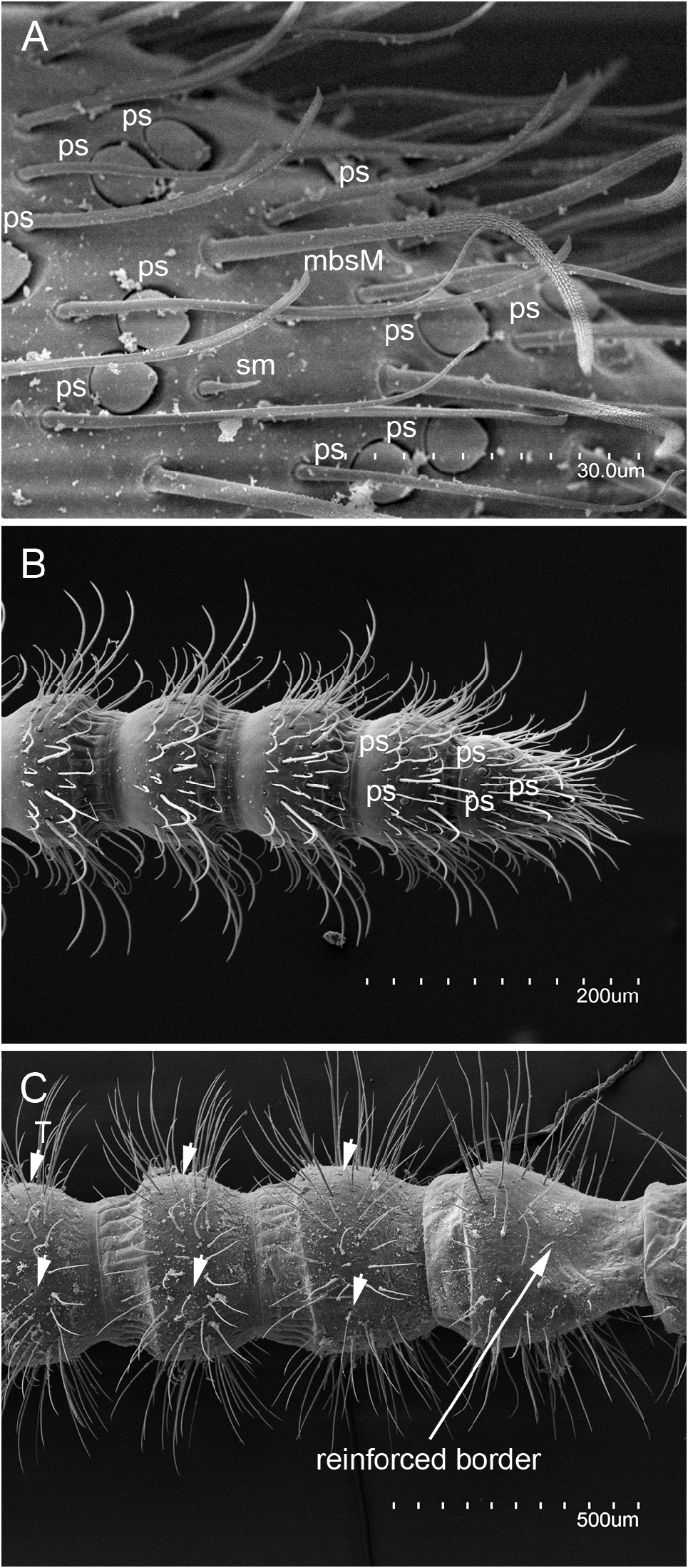
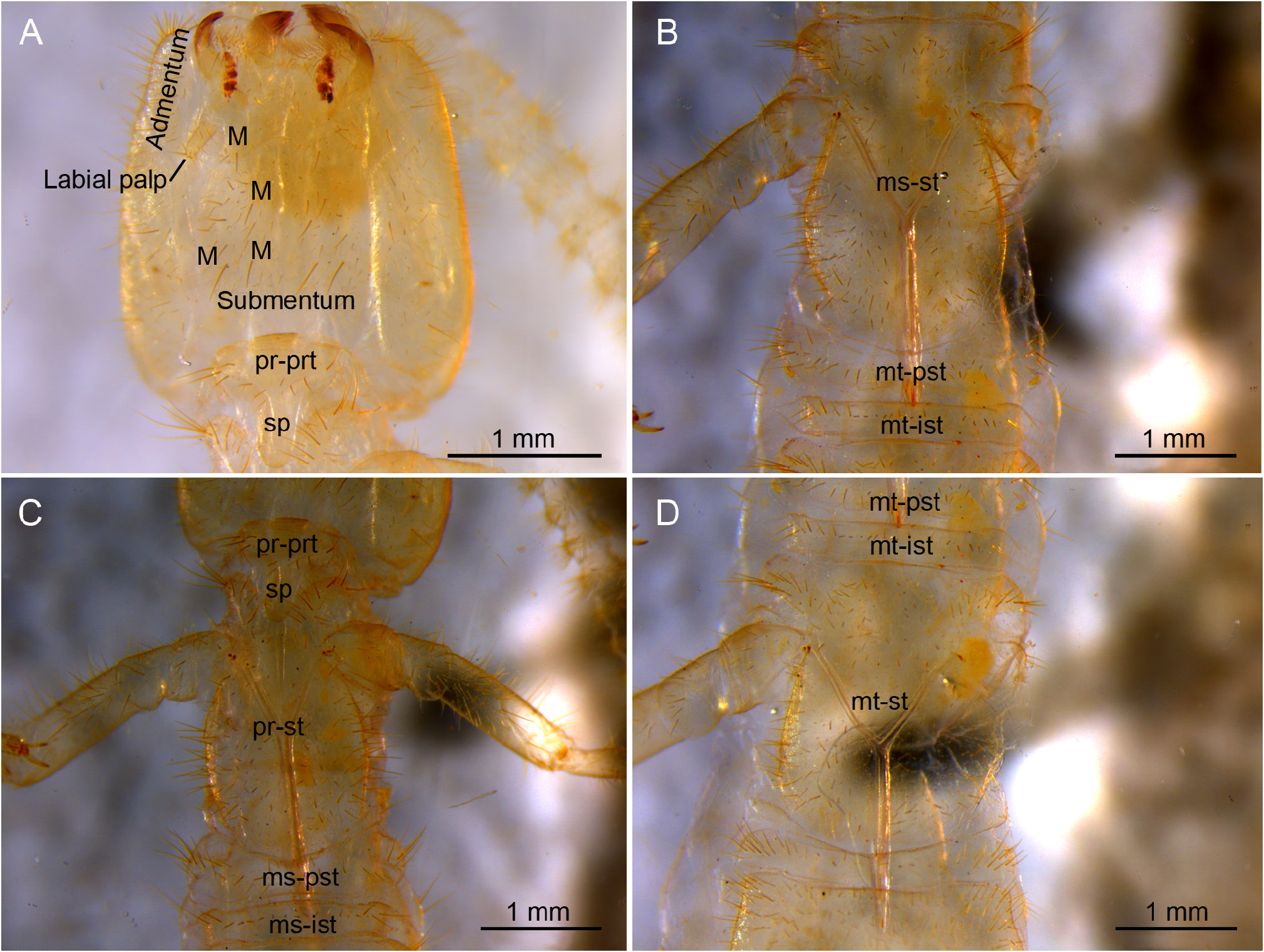
HEAD. Antenna length 9.5 mm, 0.35× length of body, with 49 antennomeres; basal antennomere short, followed by two slightly longer antennomeres; antennomeres I−IV with reinforced borders visible on ventral side ( Fig. 16C View Fig ); medial antennomeres 1.1× wider than long ( Fig. 16B View Fig ). All antennomeres with M and a few ms setae, plus three whorls of mbsM ( Fig. 16A‒C View Fig ). Trichobothria present on antennomeres IV‒VII in a 3/4/4/4 pattern, with a trichobothria in central position ( Fig. 16C View Fig ). Apical antennomere with 16‒18 placoid sensilla distributed in three irregular groups; penultimate antennomere with two placoid sensilla ( Fig. 16A‒B View Fig ). Dorsal and ventral side of head with abundant sM and ms uniformly distributed and apparently without M ( Fig. 17A View Fig ); on ventral side: submentum with 2+2 M in anterior position plus 2+2 M in posterior position, admentum with 3+3 M, mentum at base of labial palps with 1+1 M; external lobes of mentum with abundant sM and the pair of exertil vesicles visible ( Fig. 18A View Fig ). Labial palp short, length 0.24 mm, 3.2× as long as wide, with one proximal sM and four medial and distal sM plus several ms. Lacinia falciform, well sclerotized, all five laminae large and pectinate.
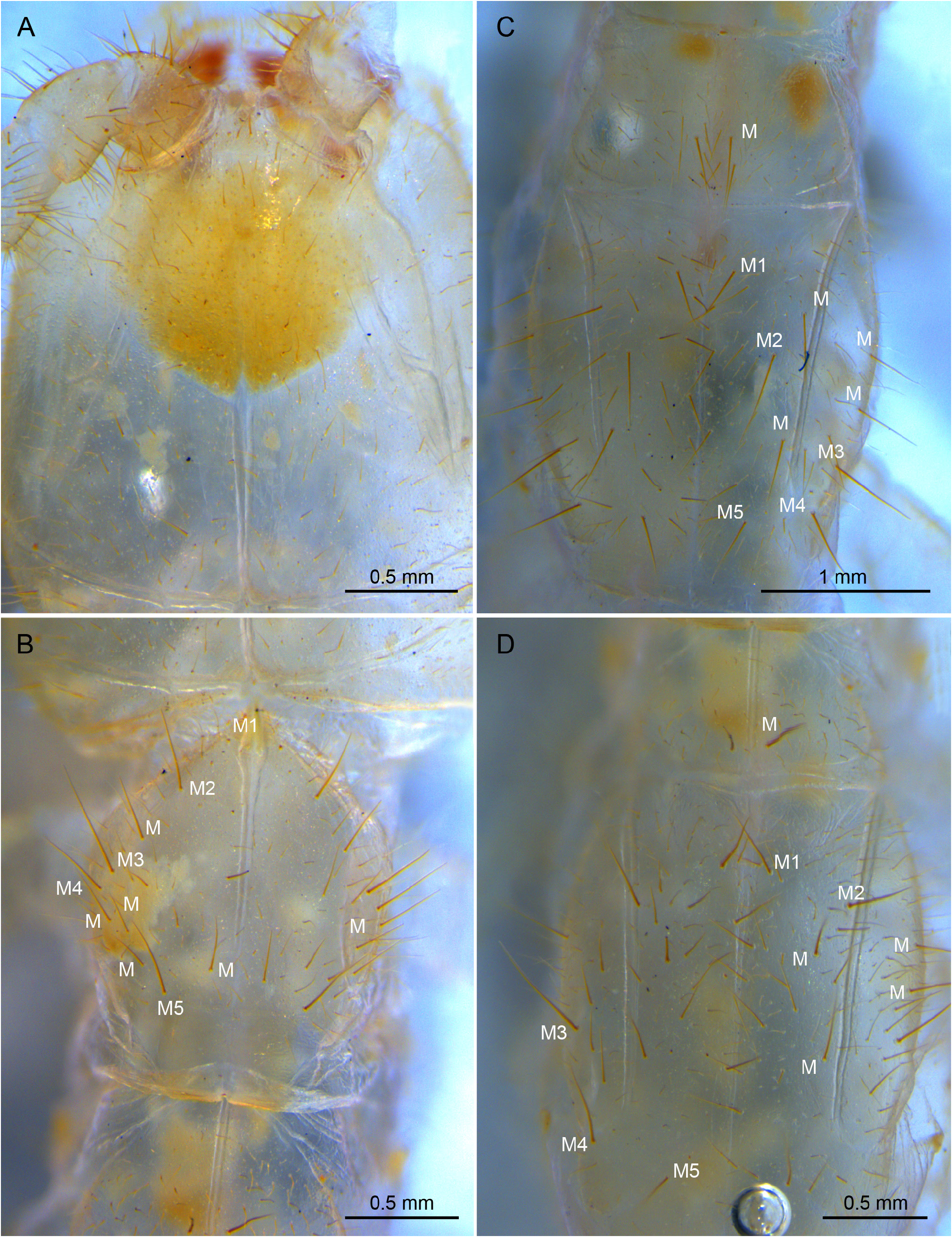
THORAX. Thoracic segments elongate, with extra M, several sM, and abundant ms uniformly distributed. Pronotum with 5+5 M1−5 plus extra 6+5 M; prescutum of mesonotum with 1+1 M; mesonotum with 5+5 M1−5 plus extra 4+4 M; prescutum of metanotum with 1+1 M, metanotum with 5+5 M1−5 plus extra 4+4. ( Fig. 17B‒D View Fig ). Thoracic sternites, intersternites, and presternites defined, with ms, sM and M ( Fig. 18 View Fig A−D). Pro-presternites and pro-, meso- and metasternites with strong internal Y-shaped cuticular structures (furcisternites) ( Barlet & Carpentier 1962), only in pro-presternites the prolongation of posterior branch (spine) is visible on the surface ( Denis 1949). Pro-presternum with no clearly defined limits, spine with apparently 3+3 M and some sM; prosternum with about 70 M or sM well distributed in a variety of shapes; meso-poststernum with 10+10 M-sM; meso-intersternum with 7+7 M-sM; mesosternum with about 100 M-sM; meta-poststernum with 16+16 M-sM; meta-intersternum with 10+10 M-sM; and metasternum with about 160 M-sM. Legs slightly short, hind leg 4.6 mm long, reaching third abdominal segment. Femur-tibia-tarsus articulations with a row of sM; coxa with 14 ventral M-sM; trochanter with 10 M-sM; femur with 24 ventral M-sM and 16 dorsal M-sM; tibia with 20 M-sM; tarsus with 30 M-sM plus two ventral rows of 5 and 6 thick setae. Pretarsus with two short, thick, unequal claws, and a rounded medial unguiculus.
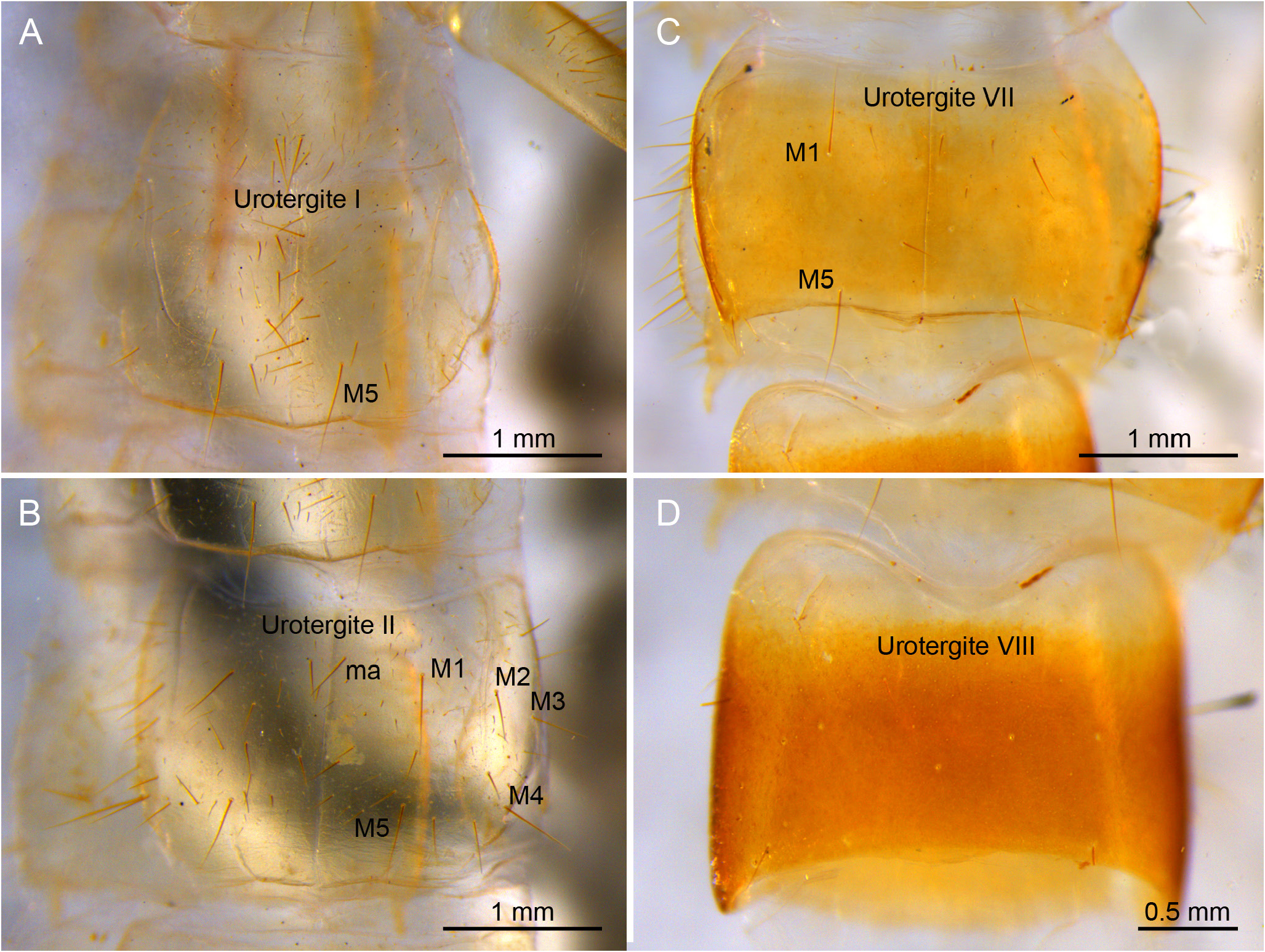
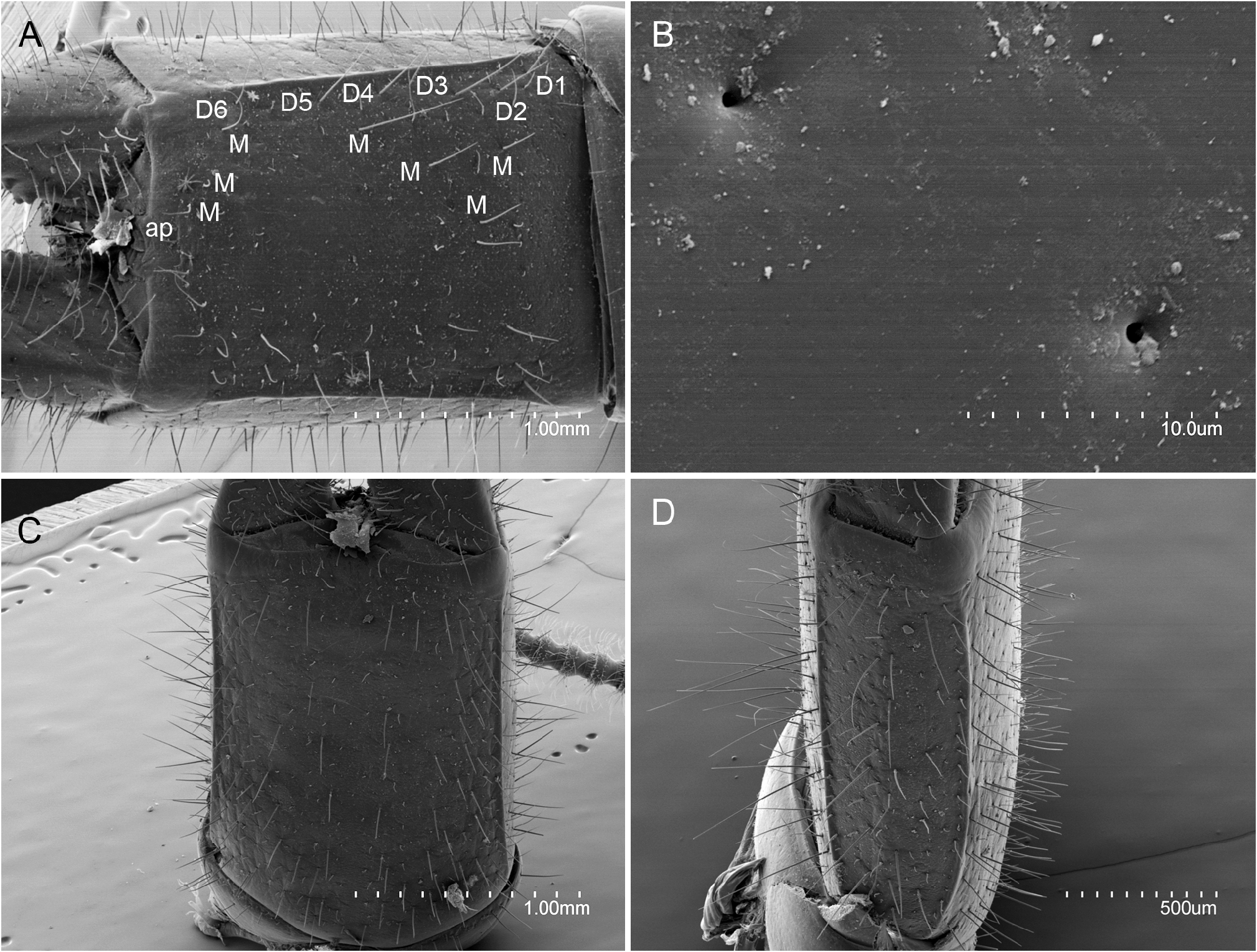
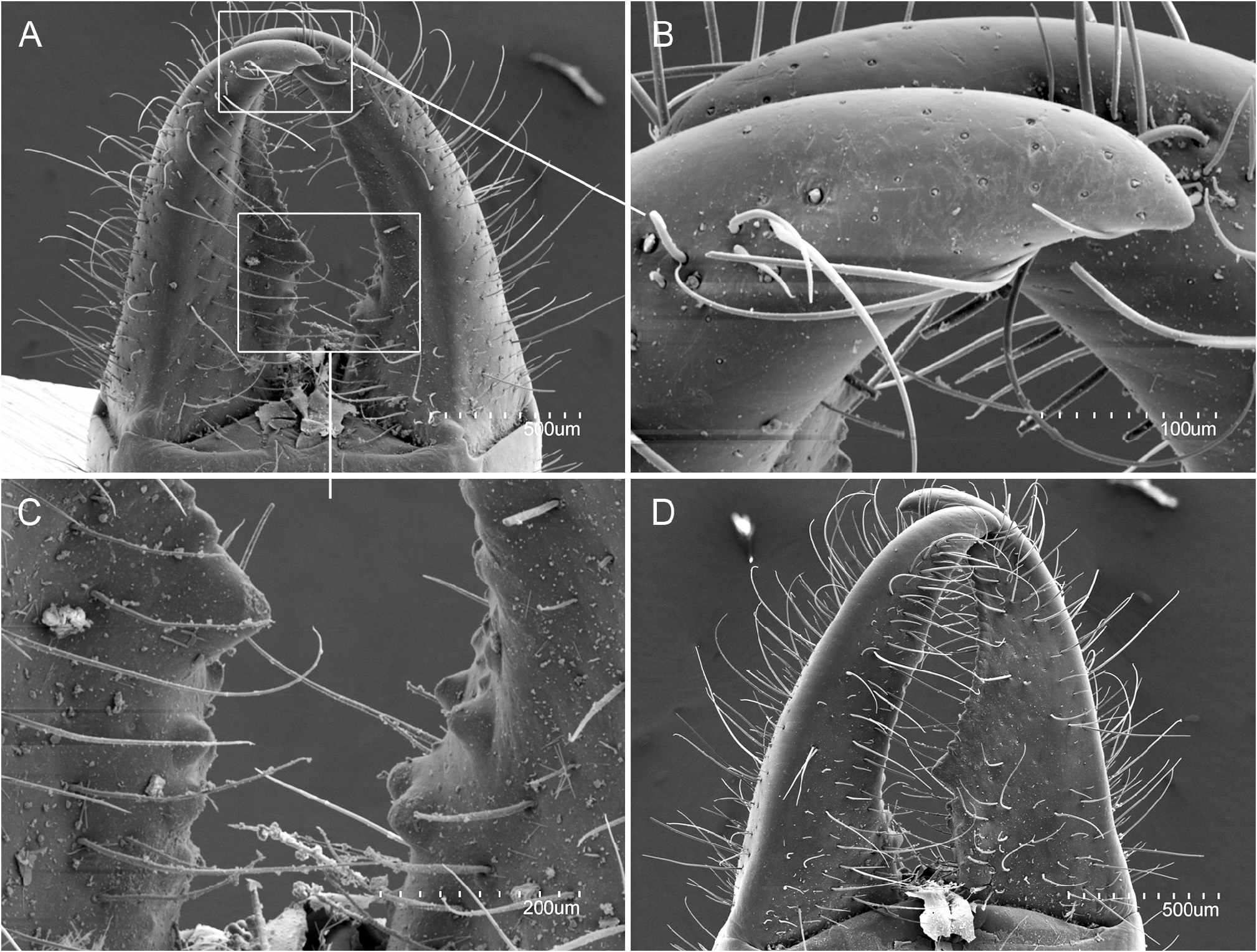
ABDOMEN. Abdominal tergites with a few ms and sM plus several M or sM. Prescutum of urotergite I with 1+1 M, scutum with 1+1 M or sM (ma), 1+1 M1, 1+1 M5 and 2+2 medial intermediate M; urotergite II with 1+1 M(ma), 2+2 M1−2, 2+2 M4−5, 1+1 medial intermediate M and 1+1 lateral intermediate M; urotergites III−VII with 1+1 M or sM (ma), 5+5 M1‒5, 1+1 medial intermediate M and 1+1 lateral intermediate M; urotergite VIII with 2+2 M; urite IX with 12+12 ventral M ( Fig. 19 View Fig A−D). Urite X 1.5× as long as wide; with distinctly marked carinae; carinae with subparallel margins; dorsal side with 6+7 intracarinal D1−6 M plus 2+2 lateral M between D2, 1+1 medial M and 1+1 lateral M between D3, 1+1 lateral M between D3−4, and 3+3 M between D6; acropygium rounded; lateral side with 5 rows of 8−10 M; ventral side with 4+4 rows from lateral to central with 7−8 M ( Fig. 21A‒D View Fig ). All tergites with blunt, slightly rounded posterolateral angles ( Figs 19 View Fig C−D, 20C‒D). Urosternite I ( Fig. 20 View Fig A−B) with scarce ms and abundant sM indistinct from M; sM more abundant at the narrow anterior portion of lateral subcoxal organ, with about 90 sM each. Median glandular organ with several tiny setae ( Fig. 19E View Fig ). Lateral subcoxal organ with three rows of short glandular setae (GS) (about 240 GS) and one posterior row of about 60 sensory setae (SS); lateral subcoxal organ occupying 0.36× of interstylar width/area; GS/st1 and SS/st1similar= 0.14 ( Fig. 20A‒B View Fig ); urosternites II‒VII with scarce ms and abundant sM undifferentiated from the thick and bunt apex shape M ( Fig. 20C‒D View Fig ). Cerci asymmetric, strong, well-developed, length 1.4 mm, rectilinear along the proximal half and curved in the distal half, becoming a large hook towards apex; heavily sclerotized with external dorsal and ventral carinae arising from dorsal and ventral acetabular articulations; ventral carinae reaching apex and dorsal carinae before the end ( Fig. 22A‒D View Fig ). Right cercus with proximal tooth pointed; predental margin with two rows of 3+3 round denticles; postdental margin with a row of 16 small round denticles reaching near the hook. Left cercus toothless, proximal margin with three rows of 4+3+4 denticles: superior and inferior round rows with denticles, intermediate row with tiny round denticles; medial and distal margin with 12 small round denticles ending before the hook. Right cercus with 18, 32, 30 dorsal, lateral, and ventral M; left cercus with 19, 30, 30 dorsal, lateral, and ventral M; both with a few sM and ms, plus campaniform sensilla regularly distributed on internal margins and at the hook ( Fig. 22B View Fig ).
Taxonomic affinities
In 1929, Silvestri described a new genus and species, Opisthjapyx seurati Silvestri, 1929 , from the central Sahara at the Oued Tezzeït in the south of Algeria. This species is well-defined by its abundant macrosetae (M) along the body, with 14+14 M on the pronotum and 15+15 M on the mesonotum and metanotum. Additionally, it exhibits urosternites with abundant macrosetae and double rows of denticles in both cerci. Opisthjapyx naledi sp. nov., found in a South African cave, exhibits several differences from O. seurati . Four of these differences can be attributed to cave life: a larger body size (27.5 mm compared to 17 mm in O. seurati ), longer antennae (0.35 ratio of body length to antenna length compared to 0.23 in O. seurati ), 49 antennomeres (compared to 36 in O. seurati ), and a greater number of placoid sensilla on distal antennomeres. Two other distinguishing features of O. naledi include the number of thoracic macrosetae (M), with 11+10 M on the pronotum and 9+9 M on the mesonotum and metanotum (compared to 14+14 and 15+ 5 in O. seurati ), as well as the presence of three rows of denticles instead of two rows as seen in O. seurati .
Habitat
The single known specimen of Opisthjapyx naledi sp. nov. was found in the Villa Louisa Cave. This cave is located in the Cradle of Humankind, UNESCO World Heritage Site (Gauteng Province, South Africa). The region is highly diverse in species, as it is associated with the Rocky Highveld Grassland. The rainy period is during the hot summer months, with an annual average of 700 mm. The caves in the area are mainly developed in dolomitic bedrock, associated to the Monte Christo Formation (Malmani Subgroup, Transvaal Supergroup) ( Dirks et al. 2015). Despite extensive search efforts for invertebrates in the cave, only one single specimen was found, thus indicating the rarity of this species. The specimen was found in a deeper, moist, and aphotic area within the cave. The specimen was found under a rock, sheltering in a small chamber, likely dug by itself ( Fig. 23D View Fig ). After removing the rock, the japygid remained immovable and only left the soil chamber after it was disturbed with a brush tip; it started to run in search of another shelter. However, it did not react to the light, thus not presenting any phototaxy.
The Villa Louisa Cave’s external area is highly altered, especially by deforestation. Furthermore, near the cave’s entrance (approximately 70 meters), there is an asphalt factory posing a pollution threat. The caves’ entrance has small bush patches surrounded by grass ( Fig. 23A View Fig ). Its interior is highly impacted due to the removal of calcite deposits, and the conduits and floor were severely altered in part by walls that were built inside it ( Fig. 23B View Fig ) and tyres that were installed to serve as a staircase in the entrance chamber ( Fig. 23C View Fig ). The cave still receives local visitors and presents signs of religious use (candles and jars).
The risk of contaminants originating from the farms and factories near the cave certainly represents a major concern for this species’ conservation. Furthermore, we need further samplings in the area to determine the actual distribution of this rare species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Japygoidea |
|
Family |
|
|
Genus |