Cautethia gossi Miller, Matthews, and Gott, 2022
|
publication ID |
https://doi.org/ 10.5281/zenodo.7167978 |
|
publication LSID |
lsid:zoobank.org:pub:D0590B45-FCBC-4411-B50B-A80940C5EA28 |
|
persistent identifier |
https://treatment.plazi.org/id/039D8797-FFAF-FFFC-FF6F-FC3010A3FC2E |
|
treatment provided by |
Felipe |
|
scientific name |
Cautethia gossi Miller, Matthews, and Gott |
| status |
sp. nov. |
Cautethia gossi Miller, Matthews, and Gott , new species
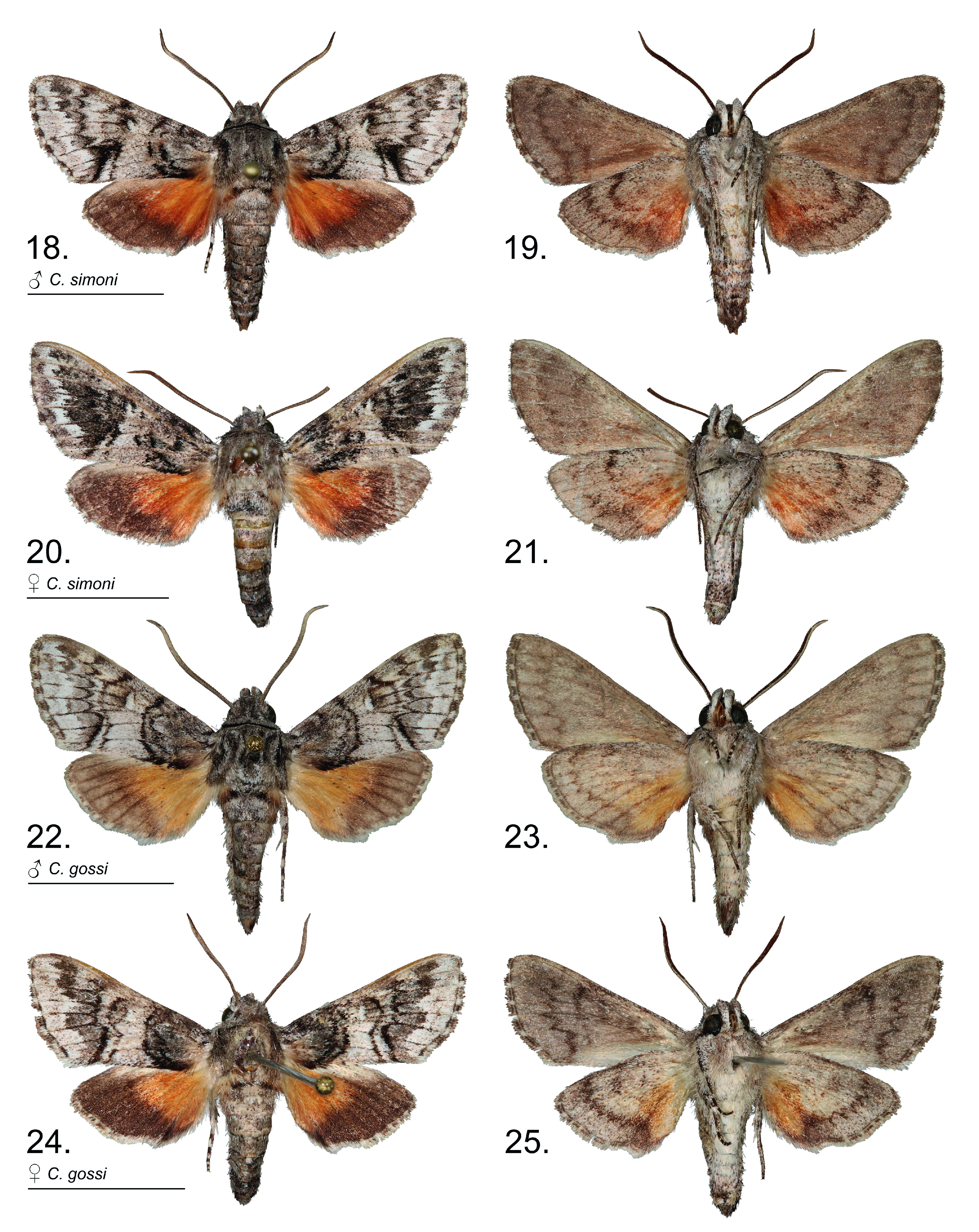
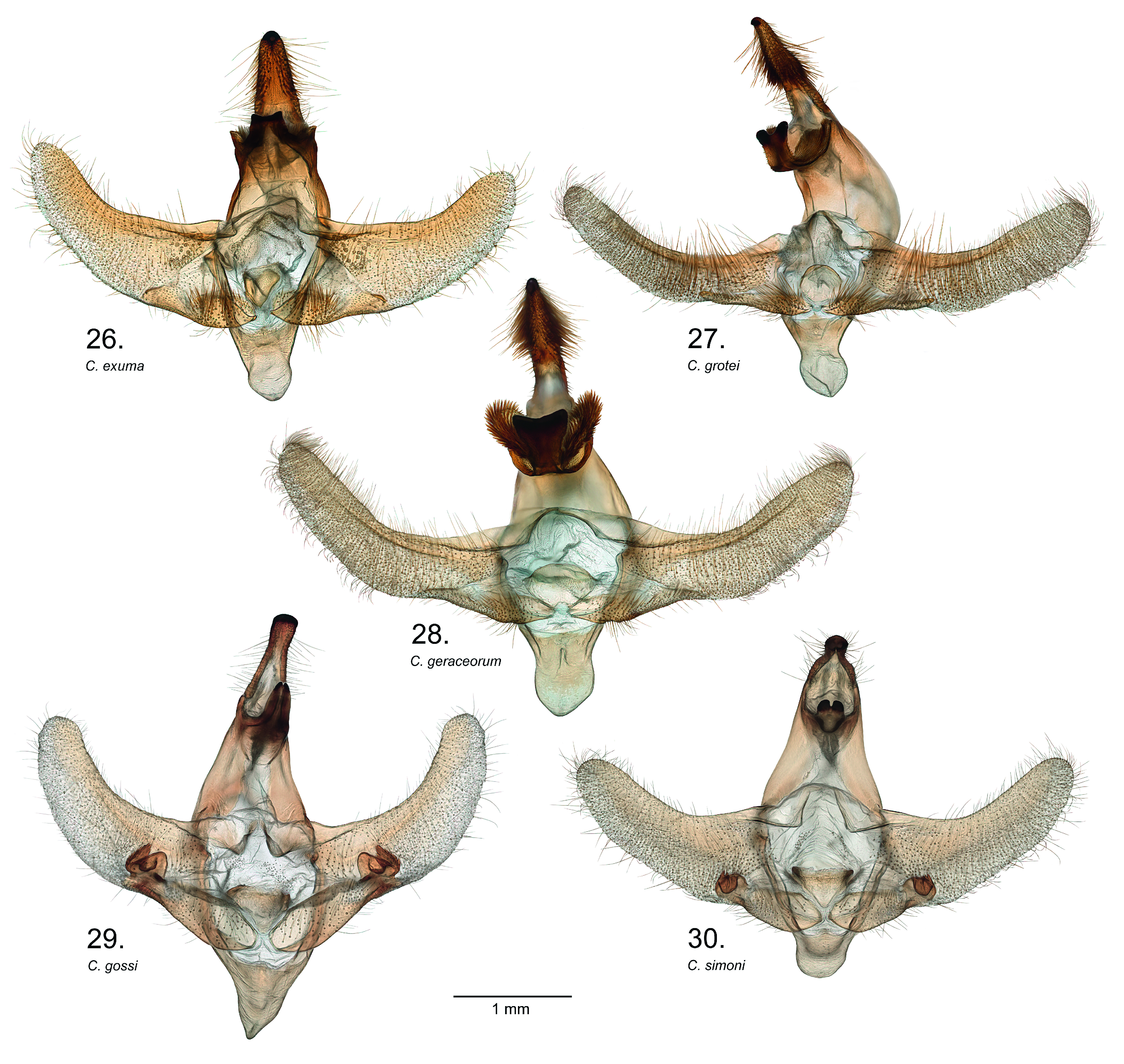
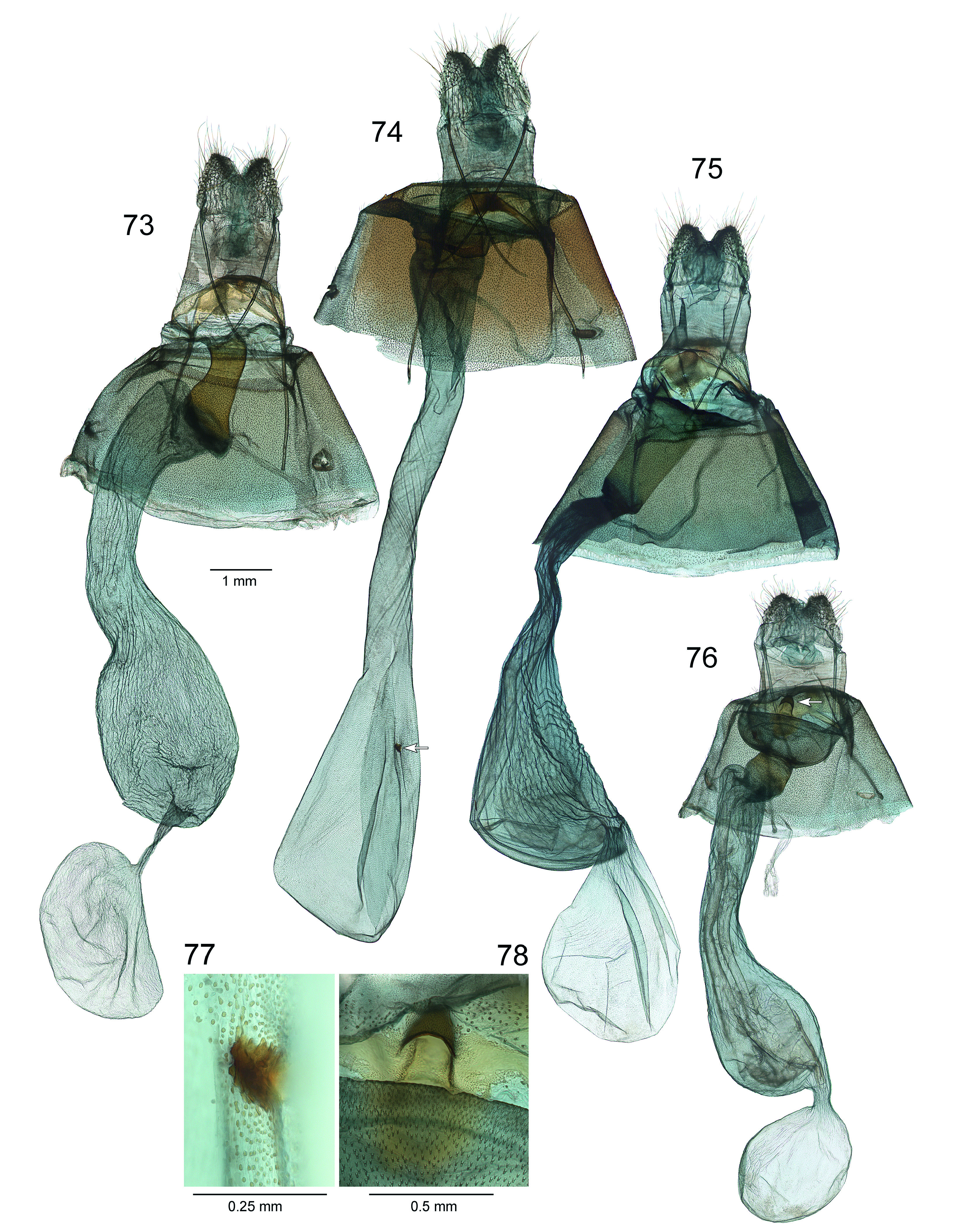
Fig. 22–25 View Figures 18–25 , 29 View Figures 26–30 , 39, 40 View Figures 31–41 , 50, 51 View Figures 42–57 , 63 View Figures 58–67 , 76, 78 View Figures 73–78
Diagnosis. Wing patterns of this species are similar to C. simoni except in the forewing antemedial lines where the basal line is indented within cell Cu 2 –A 2+3 as opposed to appearing parallel with the more distal antemedial line. In males, the costal half of the postmedial area tends to be narrower than in C. simoni . The species is best distinguished by genitalia characters. In males, the saccus is tapered to a point ( Fig. 29 View Figures 26–30 ) as opposed to rounded in congeners. The sacculus bears a uniquely shaped bilobed terminal sclerotized process ( Fig. 63 View Figures 58–67 ), and the gnathos lacks basal lobes and the apex is tapered to narrow notched tip. Female genitalia are unique from congeners in having the antrum divided into a wide posterior half and a narrower anterior half, as well as having a sclerotized cup-like receptacle on the lamina postvaginalis ( Fig. 78 View Figures 73–78 ).
Description (male). Based on the holotype and eight male paratypes. Head. Front, palpi, and antennae as in C. simoni . Thorax. Dorsum similar to C. simoni except tegulae with fuscous scaling heavier at base and not distinctly diverging into traces along margins. Venter white, mixed with drab and buff. Legs banded as in C. geraceorum and C. simoni . Forewing. Length 13.5–14.5 mm, x= 14.0 ± 0.5 mm (n = 9), holotype 14.5 mm. Basal and subbasal areas mottled dark gray, together tending to appear darker than in C. simoni and contrasting paler medial area. Antemedial lines fuscous with basal of the pair indented at middle of cell Cu 2 –A 2+3. Medial area white with scattered drab scales, with less drab than in C. simoni and with area distad of discal cell tending to be broader and paler than in C. simoni , and with drab grading to white from discal spot. Drab to fuscous trace on veins M 1 –Cu 1 distinct. Discal spot oblong, pure white, distally margined in fuscous. Postmedial area white with scattered drab scales, similar in shade to medial area; tending to be narrower near costa than in C. simoni , nearly contiguous from costa to anal margin with constriction at apex of tornal dash. Tornal dash with fuscous part formed from postmedial line bolder than adjacent medial line, tending to be of uniform width (not widened or blurred at tornal dash apex as in C. simoni ). Submarginal area with subapical triangle and area bordering postmedial line drab, remaining subterminal area tending to be white, flecked with drab scales and paler than in C. simoni ; without distinct elements of subterminal line separating terminal area. Venter of forewing variable as in C. simoni . Hindwing. As in C. simoni except basal two-thirds on dorsum clay color as opposed to ochraceous tawny in fresh specimens. Abdomen. Dorsum similar to C. simoni . Lateral patch of fuscous scales on A4 sometimes trailing to dorsum but not as developed as in C. grotei ; a smaller patch laterad on A6. Venter white or cream buff with scattered buffy brown scales.
Male genitalia (n = 2). Tegumen relatively narrow in lateral aspect. Uncus exceeding gnathos in lateral aspect, distally tapered to a blunt sclerotized point ( Fig. 51 View Figures 42–57 ), setae minute to short, sparse. Gnathos without basal lobes, apex fused, curved slightly dorsad, terminus darkly sclerotized, with a few minute teeth or scobinations. Distal aspect of gnathos tapered to a narrow apex with a minute median notch. Valvae strongly curved toward dorsum, slightly wider at base, distal ends rounded. Sacculus ( Fig. 63 View Figures 58–67 ) terminating in distinct bilobed sclerotized process consisting of a smooth protruding basal flange partly overlapping a strongly dentate terminal lobe. Juxta lightly sclerotized. Saccus apex acute. Phallus ( Fig. 39 View Figures 31–41 ) slightly constricted between anellus and apex, dorsal apex darkly sclerotized and forming spine-like process with a sharp clear tip ( Fig. 40 View Figures 31–41 ). Vesica with broad membranous area followed distally by a spiculate lateral lobe and tubular terminal part as in C. simoni but with a distinctive proximally projecting ventral lobe near base.
Description (female). Based on three female paratypes. Forewing length 12.0–14.0 mm, x= 13.2 ± 0.5 mm (n = 3). Overall, very similar to males except medial area distad of discal cell spot darker, similar in shade to basal area. Postmedial area also slightly darker from costa to apex of tornal dash. Submarginal area with dark gray apical triangle more or less contiguous with dark gray band along postmedial line and including tornal dash. Hindwing as in males. Abdomen with lateral fuscous markings less distinct than in males.
Female genitalia (n = 1). Papillae anales moderately setose with longest setae about half length of papillae anales. Apophyses posteriores length about 2.5× that of papillae anales. Free part of apophyses anteriores length about 1.5× that of papillae anales. Antrum constricted near middle, subdivided into a broad concave pocket posterad and a cylindrical collar anterad. Lamina postvaginalis with a small moderately sclerotized cup-like receptacle ( Fig. 78 View Figures 73–78 ). Inception of ductus seminalis near juncture of antrum with ductus bursae. Ductus bursae wider than anterior part of antrum, grading into pyriform corpus bursae, width similar to posterior part of antrum. Appendix bursae present, round, with short, stout stalk-like connection to anterior right end of corpus bursae, and of similar diameter.
Types. HOLOTYPE. ♂ - with the following labels: ‘ BAHAMAS: Great Inagua │ 0.95 mi. SE of lighthouse │20.926944°, −73.661111°│ 26.vii.2014 │M.J. Simon & G. Goss’ [white printed]; ‘Bahamas Survey│MGCL Accession │No. 2014-21’ [white printed]; ‘ HOLOTYPE ♂ │ Cautethia gossi │J.Y. Miller │D.L. Matthews │ R.J. Gott’ [red printed]; ‘MGCL 242737 │ McGuire Center for Lepidoptera │& Biodiversity, FLMNH, UF’ [green printed with barcode]. The holotype is deposited at MGCL. PARATYPES. 8 ♂, 3 ♀ - BAHAMAS: Great Inagua: 0.95 mi. SE of lighthouse, 20.926944°, −73.661111°, 26.vii.2014, MJS, GJG, MGCL Acc. 2014-21 (2 ♂, prep. DM 2131) MGCL 246338 (LEP-65158), 246339; 1 mi. ESE of lighthouse, 22.934722°, −73.661944°, 23.vii.2014, MJS, GJG, MGCL Acc. 2014-21 (1 ♂) MGCL 240269; Man of War Bay nr. Calf Pond, 20°56’N, 73°40’W, 9–12.vi.2007, L.D. Miller, JYM, MJS, mesic trop. forest, MGCL Acc. 2007-9 (1 ♂, prep. DM 2136) MGCL 231522, (3 ♂) MGCL 231520, 231521, 231523; 1.3 mi. NNE of Morton dock, 21.066111°, −77.638056°, 27.vii.2014, MJS, GJG, MGCL Acc. 2014-21 (1 ♂) MGCL 242667, (1 ♀, prep. DM 2137) MGCL 242680; 3 mi. SW of Morton dock, 21.022222°, −73.685556°, 27.vii.2014, MJS, GJG, MGCL Acc. 2014-21 (1 ♀) MGCL 234858 (LEP-65156); 7.4 mi. N of airport, 21.082516°, −73.641644°, 24.vii.2014, MJS, GJG, MGCL Acc. 2014-21 (1 ♀) MGCL 242696.
Life history. Unknown.
Distribution. Known only from Great Inagua.
Etymology. This species is named in honor of one of the collectors of the type series, Gary Jack Goss, who has been a dedicated member of the Miller Bahamas field team, assisting not only with collecting but also field photography of moths. Goss also has a past connection to the type locality, having studied pollination biology of orchids of the genus Encyclia Hook. on Great Inagua during the early 1970s.
Results – Barcode Analyses
The deeper level relationships among taxa are not clear based on molecular data collected for this study due to the limitations of using only COI barcodes ( Rubinoff and Holland 2005; Talavera et al. 2022). We do note, however, that our putative outgroup Himantoides undata (Walker) along with Cautethia carsusi Haxaire and Schmit and C. calezae Haxaire and Melichar , were found to be further apart from the other Cautethia species examined ( Fig. 79 View Figure 79 ) and this separation may correspond with morphological characters such as female genitalia once these are more completely known for the genus. Within the genus, there is moderate barcode support for a clade composed of C. simoni , C. gossi , and C. noctuiformis . There is weak barcode support for a clade containing C. spuria , C. yucatana , C. grotei , and C. exuma . A sister relationship of C. geraceorum to C. grotei + C. exuma is also weakly supported. There is likewise weak support for the sister relationship of C. grotei and C. exuma .
The identities of existing COI sequences for C. spuria and C. yucatana are unclear and need further examination. These sequences are nearly identical and may only represent one of the two species. Males of these two species can be separated without dissection by examining the tip of the uncus. Morphological examination of previously sequenced specimens may help resolve the identity of these problematic sequences. Another complication is the possible synonymy of C. simitia with C. yucatana . Additional molecular work sampling primary types may be helpful in resolving the identities of reported sequences and questions of synonymy.
In addition to morphological characters delimited in the species accounts, there is strong evidence seen in our pairwise distance matrix ( Fig. 80 View Figure 80 ) and neighbor joining tree ( Fig. 79 View Figure 79 ) to support the description and naming of the three new Lucayan species based on COI barcodes. Barcodes indicate the three species are distinct from each other and from other Cautethia in the region. First looking at C. gossi , there is a 2.2% difference from C. simoni , a 3.1–3.3% difference from C. n. noctuiformis , and a 3.9–4.1% difference from C. noctuiformis bredini Cary. Considering C. simoni , along with the noted difference from C. gossi , there is a 2.9–3.1% difference from C. n. noctuiformis and 3.7–3.9% difference from C. n. bredini. For C. geraceorum , we found a 3.5–3.9% difference from C. exuma and a 2.0–2.7% difference from the clade containing C. g. apira, C. g. bahamensis, and C. g. grotei . In addition, C. geraceorum differed from C. g. jamaicensis by 3.7–4.1% (see additional comments below).
There is more work to be done with C. grotei throughout its range, as the relationships among subspecies are not confidently supported by the neighbor joining tree and pairwise distance matrix. While outside the initial scope of our study, an unexpected result is strong evidence that the subspecies C. grotei jamaicensis is distinct at the species level from the other C. grotei subspecies as well as C. exuma and should be addressed as a separate study. Pairwise distances were 2.6–3.7% between C. g. jamaicensis and C. g. apira + C. g. bahamensis + C. g. grotei . Potential specimens of C g. hilaris were not confidently identified by wing patterns nor sequenced. In addition, further study of the C. grotei bahamensis holotype is necessary to determine its identity and confirm whether an incorrect barcode was published with its description (see comments in Materials and Methods).
The use of the 2% COI barcode gap to delimit species in the genus Cautethia is supported by previously described species having greater than 2% barcode gaps, and the new species described herein, initially noticed by morphological characters, are supported with barcode gaps greater than 2%. We view our attempt to analyze barcodes as an initial study to encourage further exploration of the genus Cautethia for additional undescribed species and to clarify relationships with additional data from multiple sources, including molecular, morphological, ecological, and biological, as described by de Queiroz (2005) with the unified species concept.
List of Cautethia Taxa and Type Localities
Cautethia carsusi Haxaire and Schmit, 2001 ; TL - Pedernales, Dominican Republic
Cautethia insolita Haxaire, 2016 ; TL - La Vega, Dominican Republic
Cautethia calezae Haxaire and Melichar, 2016 ; TL - Bosque Estatal de Susùa, Puerto Rico
Cautethia noctuiformis ( Walker, 1856)
C. noctuiformis noctuiformis ( Walker, 1856) ; TL - St. Domingo, Dominican Republic
C. noctuiformis bredini Cary, 1970 ; TL - English Harbor, Antiqua, British West Indies
C. noctuiformis choveti Haxaire 2002 , Saint Barthélemy, Lesser Antilles
Cautethia gossi Miller, Matthews, and Gott , new species; TL - Great Inagua, Bahamas
Cautethia simoni Miller, Matthews, and Gott , new species; TL - Mayaguana Island, Bahamas
Cautethia spuria (Boisduval, [1875]) ; TL - Mexico
Cautethia yucatana Clarke, 1919 ; TL - Izamal, Yucatan, Mexico
Cautethia simitia Schaus, 1932 ; TL - Simiti, Colombia
Cautethia geraceorum Miller, Matthews, and Gott , new species; TL - San Salvador Island, Bahamas
Cautethia grotei Edwards, 1882
C. grotei grotei Edwards, 1882 ; TL - Indian River, Florida
C. grotei apira Jordan, 1940 ; TL - Grand Cayman
C. grotei bahamensis, Melichar, Řezáč, Ilčíková, 2016 ; TL - Bahamas
C. grotei hilaris Jordan, 1940 ; TL - Cayman Brac
C. grotei jamaicensis Melichar, Řezáč, and Ilčíková, 2016 ; TL - Bluefields, Jamaica
Cautethia fideli Haxaire and Melichar, 2012 ; TL - Parc National Alejandro Humboldt, Mont Iberia, Guantanamo, Cuba
Cautethia exuma McCabe, 1984 ; TL - Simon’s Point, Great Exuma, Bahamas
Keys to West Indies Species
Adult habitus images for species included in the keys but not illustrated in this paper are available in the original descriptions as follows (Haxaire 2016 – C. insolita ; Haxaire and Melichar 2012 – C. fideli ; Haxaire and Melichar 2016 – C. calezae ; Haxaire and Schmit 2001 – C. carsusi View in CoL ) or online ( Kitching 2019 – C. noctuiformis View in CoL ). Genitalia dissections are usually necessary for species confirmation. Characters for known species used in the keys below are illustrated by figures herein as well as figures referenced in literature citations. Females are completely unknown for two of the ten species occurring in the region, and for one species ( C. carsusi View in CoL ) the female paratype was not dissected by Haxaire and Schmit (2001). Representative specimens were examined for all subspecies except C. noctuiformis choveti View in CoL . Keys include only species level characters so that the nominate subspecies and all other subspecies key to species level as currently defined. Future refinements may be necessary should C. grotei jamaicensis be elevated to species level based on molecular data. Prior to using the key, Cautethia View in CoL males and females may be separated by the presence of a single stout frenulum spine in males and a set of several finer bristles in females.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |