Autogneta schusteri, Behan-Pelletier, Valerie M., 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3946.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:25788BA8-0C84-4B71-A28C-D6A922BC924C |
|
DOI |
https://doi.org/10.5281/zenodo.5684798 |
|
persistent identifier |
https://treatment.plazi.org/id/039DE80E-FF99-AF4F-FF11-FF72FB7B6F68 |
|
treatment provided by |
Plazi |
|
scientific name |
Autogneta schusteri |
| status |
sp. nov. |
Autogneta schusteri View in CoL sp. nov.
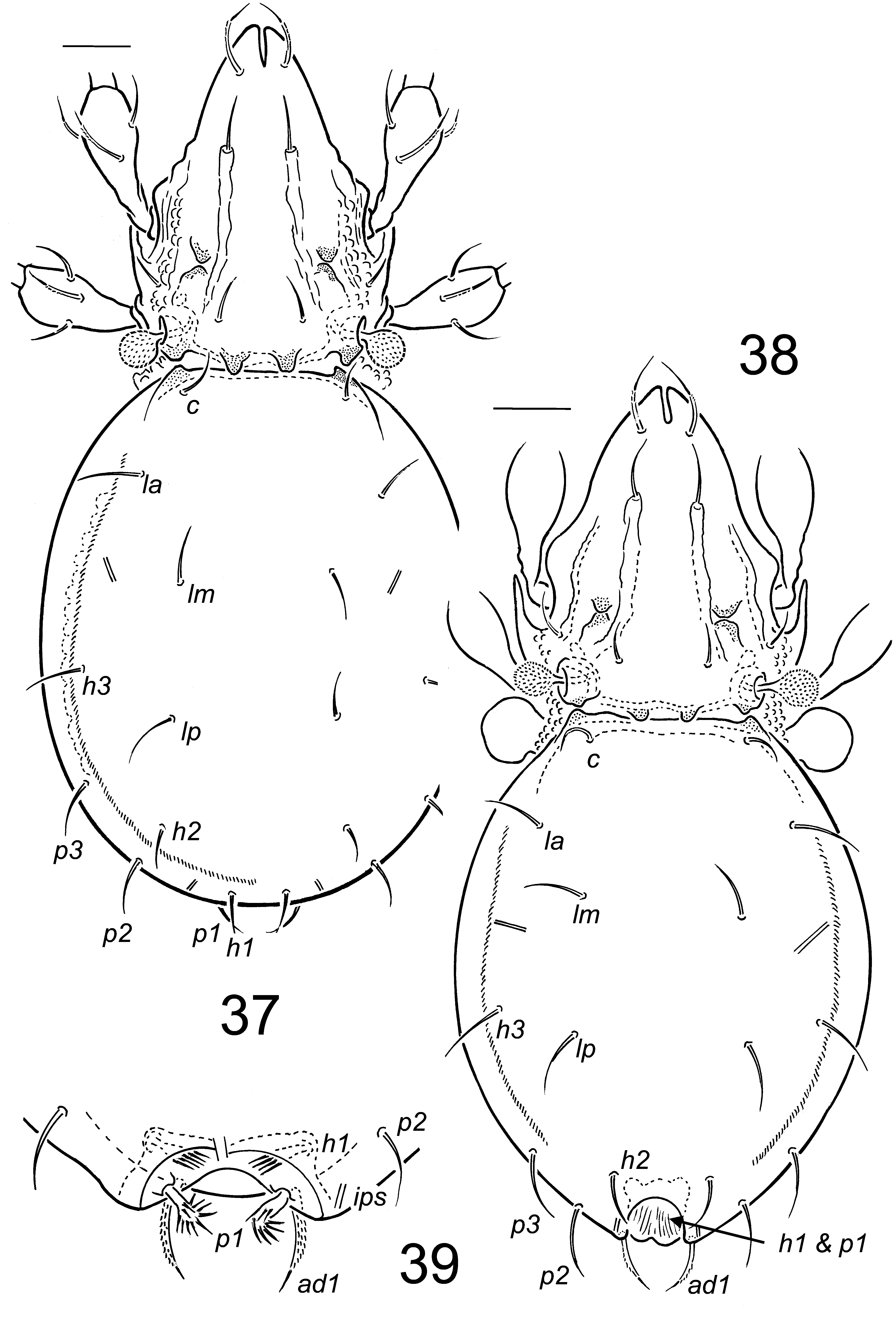
Figs 37 View FIGURES 37 – 39 –47
Material Examined. Holotype: adult male, USA, Mendocino Co., Angelo Coast Range Reserve, 39.728°N 123.645°W, 12.iii.2009 (V. Behan-Pelletier and G. Pelletier), from redwood litter in riparian area; deposited in the CNC, type number 24203. Paratypes: 6 females, 4 males with same collection data as holotype. Paratypes deposited in the CNC, USNM, and RNC.
Diagnosis. Adult. Apophyses posteriorly on prodorsum expressed as 1 pair of discrete tubercles overhanging dorsosejugal scissure. Bothridial seta capitate, about 38, globular head (about 12) with minute spicules throughout. Genital setae 6 pairs. Notogastral setae thin smooth acuminate, 20–30 long, with c shortest. Seta ad1 about 20 long, curved posteromedially, strongly barbed in male, acuminate and weakly barbed in female. Femur I without tubercles on proximal third of leg. Genua I and II with seta v’ present. Dimorphism expressed as striking posteromedial cavity (about 20 wide) with faintly porose walls, positioned between lyrifissures ips in male. Setae h1 and p1 borne internally in cavity in male; and strongly modified from those of female: thick, with tendril-like barbs, length about 20. Mutual distance h1–h1 and p1–p1 subequal; in male about 20, in female 24–27.
Description. Adults. Dimensions: Total length: females (n = 3) 330 (322–336); male (n = 1) 300. Notogastral length: females (n = 3) 213 (202–226); male (n = 1) 185.
Integument: Microtuberculate. Pleural region strongly tuberculate from base of pedotectum I to acetabulum IV. Microtubercles on trochanters III, IV and proximally on femora I–IV more evident than on other leg segments. Margin of epimeres II and IV weakly defined medially.
Prodorsum: Rostral incision about 16. Rostral setae about 19, barbed, acuminate, curved anteromedially. Costulae about 54, lateral edge defined, medial edge defined in distal third; bearing setae le anteriorly, about 15–20 long, acuminate, weakly barbed; longitudinal ridge present lateral of costula. Enantiophysis E weakly developed lateral to proximal third of costula. Apophyses posteriorly on prodorsum expressed as 2 discrete tubercles overhanging dorsosejugal scissure ( Figs 37, 38 View FIGURES 37 – 39 , 42). Seta in about 12–15, ex about 10, acuminate. Mutual distance ro–ro about 18, le–le about 21, in–in about 30. Humeral enantiophysis well-developed (Fig. 44). Bothridial seta about 38, with globular head about 12, head with minute spicules ( Figs 37 View FIGURES 37 – 39 , 44).
Notogaster: With U-shaped furrow weakly defined, outlined by microtubercles ( Figs 37, 38 View FIGURES 37 – 39 ). Notogastral setae thin, smooth acuminate (other than h1 and p 1 in male), 20–30 long, with c shortest, positioned as in Figs 37, 38 View FIGURES 37 – 39 . Dimorphism expressed as striking posteromedial cavity (about 20 wide) in male with faintly porose walls, positioned between lyrifissures ips. Setae h1 and p1 borne internally in cavity and strongly modified from those of female: thick, with tendril-like barbs, length about 20. Mutual distance h1–h1 and p1–p1 subequal; in male about 20, in female 24–27.
Ventral Region: Epimeral setae 8–14 long, tapered, slightly roughened. Genital setae 6 pairs, about 12 long, acuminate, smooth. Aggenital seta, anal and ad3, ad2 about 12 long, smooth, acuminate; seta ad1 about 20 long, curved posteromedially, strongly barbed in male, acuminate and weakly barbed in female. Setae ad1, ad2 posterior to anal plate in both sexes; seta ad3 posterior to level of iad in male, anterior to iad in female. Lyrifissure iad parallel to anterolateral margin of anal plate. Posterior border of epimere IV smooth (Fig. 45). Tubercles absent posterior to acetabulum IV.
Gnathosoma: As for genus.
Legs: Setation: leg I: 1-5-3(1)-4(2)-18(2); leg II: 1-5-3(1)-4(1)-15(2); leg III: 2-3-1(1)-3(1)-15; leg IV: 1-2-2- 3(1)-12. Femur I without tubercles positioned antiaxially. Seta v’ present on genua I and II.
Immatures: Unknown.
Etymology. This species is named in honour of the eminent acarologist, Professor Dr. Reinhart Schuster.
Remarks. Both males and females of this species are easily recognized by the capitate bothridial seta, short notogastral setae, and apophyses posteriorly on the prodorsum expressed as 1 pair of discrete tubercles overhanging the dorsosejugal scissure. Sexual dimorphism in this species consists of males with posteromedial weakly porose cavity, about 20 width, positioned between lyrifissures ips, bearing setae h1 and p1 internally in cavity. Setae h1 and p1 thick, strongly modified from that of female, with tendril-like barbs.
Gravid females carry up to 2 large eggs. Gut contents are primarily darkly pigmented fungal hyphae and spores.
FIGURES 42–47. Autogneta schusteri sp. nov. Differential interference contrast microscope images of adult males: 42, dorsal habitus, modified posterior of notogaster indicated by arrow; 43, detail of posterior of notogaster (5 layers combined); 44, prodorsum and anterior of notogaster (4 layers combined); 45, ventral plate, with arrow to modified posterior (5 layers combined); 46, 47, detail of posterior cavity of male, showing modified setae h1 and p1 (4 layers combined). Scale bar: 42–45, 47 = 20, 46 = 10.
Sexual dimorphism in Autogneta . Most oribatid mites exhibit slight sexual dimorphism in size, but strong dimorphism is uncommon ( Behan-Pelletier & Eamer 2010). In Autognetidae it was previously known only in Cosmogneta impedita and Cosmogneta kargi , which show a modified seta a’ on tarsus I ( Grandjean 1960b, 1963, respectively), and an unidentified Autogneta species from Madeira which has thickening of the integument in the humeral region of the notogaster ( Travé 1959). Herein I have shown that dimorphism also exists in other species of Autogneta , including the rather well-known type species, A. longilamellata and that it takes a form that is novel in the family. In Autogneta amnica , A. longilamellata and the 3 Autogneta species described above, sexual dimorphism is expressed as a porose region posteriorly on the notogaster in males. Although I have no direct evidence, the restriction of this porose region to the male suggests that these are secretory structures, secreting semiochemicals to enhance associative mating in these species, or to facilitate location of spermatophores by females, by marks on the substrate. A function of cuticular maintenance is also possible, as “behavior of adult males of dimorphic species may require greater production of maintenance materials” (Norton & Alberti 1997), though as these authors noted, it is less likely than that these are secreting semiochemicals. These porose regions are present despite these Autogneta species not expressing the octotaxic system of porose areas.
In this they are not unique, as other non-poronotic Brachypylina , express sexual dimorphism involving porose regions. In the amerobelbid (Ameroidea) Hellenamerus ionicus Mahunka, 1974 , porose regions are found on the ventral plate of males only ( Behan-Pelletier & Eamer 2010). In Fortuyniidae (Ameronothroidea) males only of Fortuynia atlantica Krisper & Schuster, 2008 have 4 pairs of porose regions on the notogaster, along with modifications of notogastral shape and some notogastral setae. These expressions of sexual dimorphism and those in species of Autogneta , described above, confirm that porose organs with a possible secretory function, that apparently occur independent of the octotaxic system, can express sexual dimorphism. For example, possibly secretory porose organs are found on anal plates in males only of the zetomimid Heterozetes aquaticus ( Banks, 1895) , and on the ventral plate in males only of H. minnesotensis ( Ewing, 1913) , species in which the octotaxic system is absent, presumably lost, as it is expressed in the con-familial genus Zetomimus Hull, 1916 (Behan- Pelletier & Eamer 2003). The close association of setae, often modified, with these sexually dimorphic porose regions, as is found in Autogneta flaheyi , A. schusteri , and A. amnica , suggests that these setae may contribute in some as yet undefined way to the dispersal of semiochemicals (Norton & Alberti 1997).
Species of Autogneta expressing sexual dimorphism are associated with habitats that are spatially discrete, e.g., decaying wood and fungi ( A. aokii , A. flaheyi , A. longilamellata , A. amnica ) and ferns ( A. amnica ); they are rarely found in general forest litter. Norton and Alberti (1997) noted that sexually dimorphic species generally inhabit non-soil microhabitats. This possibly explains why A. longilamellata , a species with a Palearctic and Nearctic distribution, is rarely encountered in ecological studies on soil microarthropods, and has been recorded primarily from decaying wood habitats, such as tree hollows ( Taylor & Ranius 2014), fallen logs ( Skubała & Marzec 2013), tree stumps (Skubała 2008), and decaying fungi on trees ( Maraun et al. 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |