Okapia johnstoni (Sclater, 1901)
|
publication ID |
https://doi.org/ 10.5281/zenodo.5719821 |
|
DOI |
https://doi.org/10.5281/zenodo.5719829 |
|
persistent identifier |
https://treatment.plazi.org/id/039F7D71-A96C-AD1C-081C-F80D7046DAD4 |
|
treatment provided by |
Conny |
|
scientific name |
Okapia johnstoni |
| status |
|
Okapi
French: Okapi / German: Okapi / Spanish: Okapi
Taxonomy. Equus johnstoni Sclater, 1901,
forests along Semliki River, Mundala, Democratic Republic of Congo.
This species is monotypic.
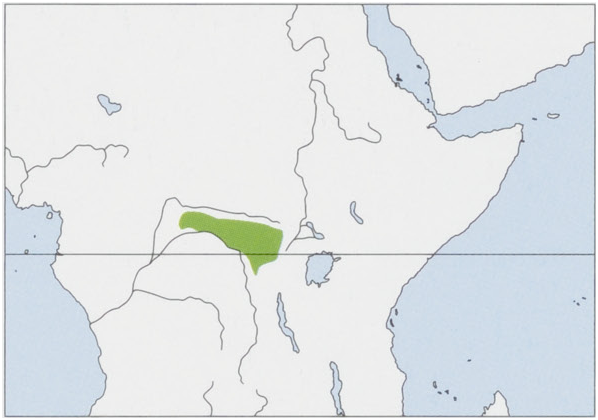
Distribution. Okapis are endemic to the rain forests of the N & NE DR Congo, and the Ituri forest in particular. View Figure
Descriptive notes. Head-body 200-210 cm, tail 30-42 cm, height from the sole of the forefoot to the crown at maturity 180 cm and to the shoulder 150—170 cm; weight 180-320 kg. Cows are noticeably taller and more massive than bulls, weighing upward of 270 kg. Almost nothing is known of Okapi anatomy and physiology. The body shape resembles that of the Giraffe, except that Okapis have much shorter necks. The pelage is dark brown-purplish on the body, with black muzzle and white to yellow stripes on the rump and forelegs. The forehead and large ears are chestnut in color. The lower legs are white with dark garters at the joints. Stripes are specific to each individual and are thoughtto help calves follow their mothers through dense rain forests, and also to break up the outline of their body, creating camouflage in the light and shade of the forest understory. With age stripes become more clear and distinct. The hair is short and oily, enabling waterproofing in a damp forest environment. The mane hairs are 4 cm long and extend from the back of the head to the rump. The tail is brown, with hair reaching almost to the hocks. There are small glands containing a waxy excretion on the front of each foot. The forefeet are used for stomping. The Okapi’s rightful place within the Giraffidae is affirmed by the skin-covered ossicones (horns) in males and the bilobate canine teeth. Males have two ossicones, which develop at 1-5 years of age and grow to be 10-15 cm long; females are hornless. As in Giraffes, as the ossicones develop they fuse with the frontal bones of the skull, a process that takes up to three years. The tips of the horns become bare of skin with time. The Okapi’s eyes are situated more laterally than in Giraffes, affording a broad field of vision, and are smaller, suggesting poorer visual acuity. At birth, calves’ eyes are surrounded by a starburst pattern, making the eyes appear larger. Hearing is exceptionally acute; the ears are large. There is a distinct nasal septum separating the nostrils. The Okapi muzzle and tongue are longer than the Giraffe’s; the pointed, black tongue is so long (up to 30 cm) that an Okapi can wash its eyelids and clean its ears. As in Giraffes, the tongue is prehensile and used for plucking tree leaves as well as grooming.
Habitat. Okapis have been able to occupy closed forest in primary or older secondary forest types. Although preferring elevations of 500-1000 m, they may be found as high as 1500 m in the eastern montane rainforests. Their range is limited to the high forests in the east, the swamp forests below 500 m to the west, savannah of the Sahel/ Sudan to the north, and open woodlands to the south. They do not occupy gallery forests or the forest islands on the savanna ecotone, nor are they to be found in the disturbed habitats surrounding larger forest settlements. They will use seasonally inundated areas while the substrate is still wet, but they do not occur in truly inundated sites or in extensive swamp forest. Tree fall gaps are their preferred foraging sites during the primary stages of regeneration.
Food and Feeding. Mature dicotyledonous browse dominates the diet of this folivorous ruminant. Okapis browse well over a hundred different species of plants of which twenty are favored, including Scaphopetalum dewevrei, Drypetes sp., and Diospyros bipendensis. They forage along well-trodden paths through the forests. They feed in small tree-fall gaps and on the edges of blowdowns. They browse by twining their long prehensile tongues around branchlets, stripping the leaves and pulling them into the mouth. They walk slowly, with a gait similar to that of the Giraffe’s, sampling leaves from right and left from palatable shrubs. They do not feed on herbaceous monocotyledons, some of which are important in the diets of chimpanzees (Pan spp.) and gorillas (Gorillaspp.), and they do not compete for browse with the relatively few other occupants of their forest biome. Duikers (Cephalophus spp.) and Water Chevrotains (Hyemoschus aquaticus) favor fallen fruits and seeds; Bongos (Tragelaphus eurycerus), African buffaloes (Syncerus spp.), sitatungas (Tragelaphus spp.), Giant Forest Hogs (Hylochoerus meinertzhageni), Bush Pigs (Potamochoerus porcus), and African Forest Elephants (Loxodonta cyclotis) all feed on foliage from canopy trees in sunlit clearings and swampy openings. Distribution of the Okapi’s food plants favors dispersed rather than congregated foraging. Nowhere is the forage abundant enough to support a herd, nor do the Okapis converge on seasonally-varying food sources, as there is continuous growth of new leaves. Females occupy forest areas that are richest in their favored food plants. There is a greater density of suitable browse in tree-fall gaps, as indicated by a change in locomotion of Okapi when feeding in gaps. When browsing in the subcanopy, their gait is steady, whereas when feeding in a tree-fall gap they walk more slowly, frequently pausing to feed on several plants from one position. Under the canopy and in gaps Okapis are selective browsers, eating only a small proportion of the species present and focussing on the youngest leaves. Food supply in the Ituri forest is patchy and variable in quality and distribution. Okapis exhibit geophagia, ingesting dirt in search of minerals.
Breeding. Male courtship is unobtrusive and cautious. Males may take the better part of a day to approach a female with overtures that include low, moaning calls. Cows may associate with two different bulls over several days. Following a gestation of 14 months, a single calf is born between August and October, and weaned after six months. Females become sexually mature at two years of age and males later, because like most ungulates in the wild, they have to become behaviorally mature to secure mating rights. The behavior of an individual female through the reproductive cycle and gestation does not change markedly. However, after parturition a considerable portion of the day is spent feeding, moving back and forth across the home range. The female travels some distance from the calf, which is hidden,visiting it a few times a day for only a few minutes each time. Calves have been observed allosuckling. A calf remains bonded with its mother for nine months. Calves make a wide range of noises, including coughs, bleats, and whistles. Males are not restricted to areas of food abundance and spend more time roaming in forest zones where palatable leaves are sparse, perhaps in an attempt to gain access to more females.
Activity patterns. Okapis are primarily diurnal and are essentially solitary, only associating to breed, except for mother—offspring pairs. Okapis are vulnerable to Leopard (Panthera pardus)attacks, several animals having been killed by Leopards during the course of one field study. Estimated population densities vary from 0-8 ind/km? to 2-3 ind/km?.
Movements, Home range and Social organization. Okapis have well-defined home ranges, the most stable of which belong to reproductive females and are in the range of 3-23-6-5 km*. Adult females have exclusive use of a territory except when nursing young. Males make brief incursions to mate. Young animals of both sexes have more restricted home ranges, overlapping with their mothers’, but eventually they emigrate. Adult males cover larger areas, sometimes more than 13 km? Males and females mark trees with an oily secretion from their skin and defecate in selected areas. They have overlapping home ranges of several square kilometres and typically occur at densities of about 1 ind/km?®. They have several methods of communicating, including pheromones from the scent glands on each foot, which leave behind a tar-like substance to signal their passage. They also mark with urine. Males defend territories. Okapis solicit allogrooming of body areas they cannot reach to groom themselves.
Status and Conservation. Classified as Near Threatened on The IUCN Red List. The Okapi was unknown to Europeans until 1901. Although it has been estimated that there are around 10,000 -20,000 individuals in the wild, this estimate is likely to be optimistic because they are shy and rarely seen. The most numerous populations of Okapi are found in the Ituri/Aruwimi and adjacent Nepoko Basin forests, and the forests of the upper Lindi, Maiko and Tshopo Basins. The species is also present in the Rubi-Tele region in Bas Uele. It was once present in the Semliki Forest of Uganda, but appears not to have survived in this region. In Virunga National Park, DR Congo, the first official sighting since 1959 was made in 2006. As of 2010, about 160 specimens are on display in some 40 zoological gardens. The name Okapi combines two words in the Congolese Lese Karo dialect, “Oka,” meaning to cut, and “kpi,” which refers to the design made on Efé arrows by wrapping the arrows with bark, making the arrows striped when they are scorched by fire. Lese legend has it that the Okapi’s stripes add to the animal’s great camouflage. The specific epithet, johnstoni , recognizes the explorer Sir Harry Johnston, who organized the expedition thatfirst acquired an Okapi from the [turi forest. Accurate population assessments are difficult in the dense forests. The future of Okapis depends on preservation of their habitat, especially the Ituri forest, an unlikely prospect given the political instability of that region.
Bibliography. Bodmer & Rabb (1992), Colbert (1938), Hart (1992, 2001), Hart & Hall (1996), Hart, J.A. & Hart T.B. (1989), Hart, T.B. & Hart J.A. (1992, 1998), IUCN/SSC Antelope Specialist Group (2008), Landsheere (1957), Lindsay (1999), Pellew (1984a).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |