Molineus lotoris, Mata-López, Rosario, 2017
|
publication ID |
https://doi.org/10.11646/zootaxa.4320.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:17Ab14E7-Da61-482E-933F-171585E64036 |
|
DOI |
https://doi.org/10.5281/zenodo.6021274 |
|
persistent identifier |
https://treatment.plazi.org/id/039F879C-FFCC-2227-FF5B-F9CAFA6062E9 |
|
treatment provided by |
Plazi |
|
scientific name |
Molineus lotoris |
| status |
sp. nov. |
Molineus lotoris n. sp.
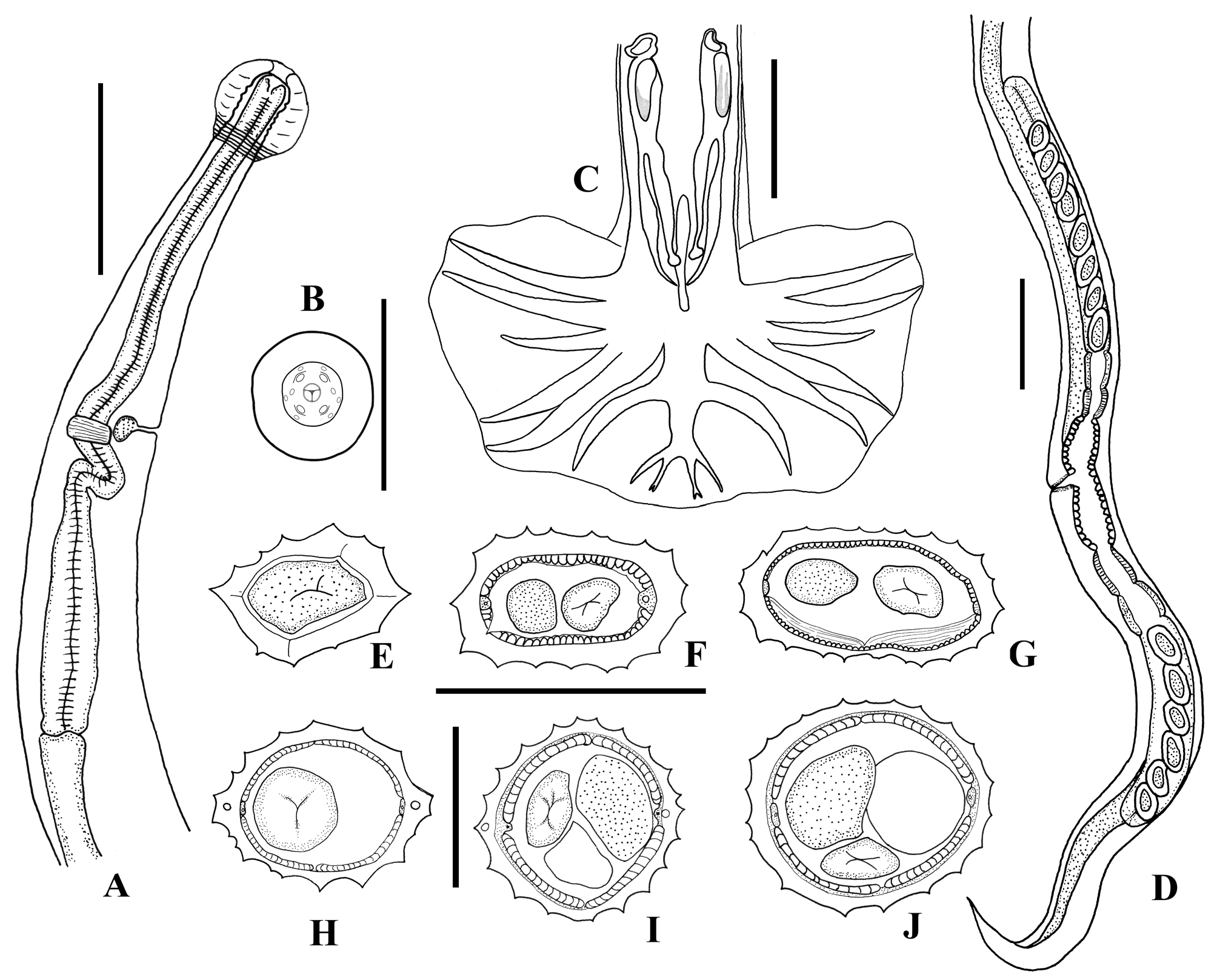
( Fig. 1 View FIGURE 1 , A–J)
Type host. Procyonidae : Procyon lotor L., Raccoon.
Type locality. Motozintla, Chiapas State, Mexico, 15°22’ 00’’N latitude, 92°15’00’’ W longitude.
Type specimens. Holotype, male (CNHE 9238); allotype, female (CNHE 9774); paratypes, 3 females, 1 male (CNHE 9309).
Site of infection. Stomach.
Prevalence and intensity. 1 of 1 raccoon infected with 11 worms.
Etymology. The species epithet refers to the specific name of the host.
Description. Nematodes of medium size, body not coiled. Females greater than males. Prominent and globular cephalic vesicle present, terminating in several transverse striations ( Fig. 1A View FIGURE 1 ). Lips absent; in apical view, 6 externolabial, and 4 cephalic papillae and 2 small amphids present ( Fig. 1B View FIGURE 1 ). Deirids not observed. Cervical groove absent. Excretory pore opening at mid-length of claviform esophagus ( Fig. 1A View FIGURE 1 ). Males with symmetrical caudal bursa type 2–1–2 ( Fig. 1C View FIGURE 1 ). Females didelphic and amphidelphic ( Fig. 1D View FIGURE 1 ). Cuticle with fine, transverse striations. Synlophe composed of uninterrupted ridges. Number of ridges variable in both sexes and at body regions. Synlophe studied in one male and two females. Cuticular ridges begin immediately after the cephalic vesicle and terminate anterior to the anus or cloaca. Ridges orientated perpendicularly to body surface. At anterior level of females, two lateral ridges of the synlophe run closely, protruding from the rest of the ridges. Number of ridges in males: 11 just posterior to cephalic vesicle ( Fig. 1E View FIGURE 1 ), 17 at mid-body level ( Fig. 1F View FIGURE 1 ), and 24 at posterior end ( Fig. 1G View FIGURE 1 ); in females, number of ridges 19–20 just posterior to cephalic vesicle ( Fig. 1H View FIGURE 1 ), 17–21 at mid-body level ( Fig. 1I View FIGURE 1 ), and 17 at posterior region ( Fig. 1J View FIGURE 1 ).
Male. Body 2.12 (1.8–2.41 ± 0.30, n=4) length by 0.05 (0.04–0.06 ± 0.005, n=4) maximum width. Cephalic vesicle 0.36 (0.34–0.39 ± 0.02, n=3) long by 0.12 (0.15–0.28 ± 0.1, n=3) wide at anterior end. Distance from anterior end to excretory pore 0.11 (0.10–0.12 ± 0.02, n=4) and to nerve ring 0.12 (0.12–0.14 ± 0.07, n=4). Esophagus 0.13 (0.12–0.16 ± 0.02, n=4) by 0.014 (0.013–0.015 ± 0.001, n=4) in maximum width. The bursa is symmetrical, covered ventrally by minute spine-like structures. The bursal ray pattern is of type 2–1–2. Rays 2 and 3 separate near base, run separately, and reach bursal margin. Rays 4 diverges at base from other lateral rays, deflected ventral, approximately l/2 length of other lateral rays. Rays 5 and 6 diverge close to base, are parallel, and reach bursal margin. Rays 8 originates approximately 1/3 of distance from base of dorsal ray, directed dorsolaterally, not reaching bursal margin. Dorsal ray thick, divided into 2 long primary branches. Each one of these, in turn, forks again into rays 9 and 10. Rays 10 distinctly bifid. External branch (rays 9) longer, curving ventrally. Spicules complex, base of handle with thickenings; equal in size, 0.10 (0.099–0.115 ± 0.008, n=4) long; blade divided approximately at 1/2 distance from proximal end into external and internal processes. Internal process slender, ending in globular swelling. External process terminating in point. Gubernaculum 0.048 (0.043– 0.052 ± 0.004, n=3) long; crosier-like, curving ventrally, with ventral projection and slight anterior protrusion ( Fig. 1C View FIGURE 1 ). Distal end terminates bluntly.
Female. Worms 2.91 (2.46–3.36 ± 0.31, n=7) long by 0.066 (0.006–0.071 ± 0.003, n=7) in maximum width, at the vulva level, tapering at both ends. Cephalic vesicle 0.043 (0.039–0.047 ± 0.002, n=7) long by 0.034 (0.032– 0.039 ± 0.003, n=7) wide. Distance to nerve ring and excretory pore from anterior end, 0.168 (0.162–0.175 ± 0.004, n=6) and 0.144 (0.143–0.147 ± 0.002, n=5), respectively. Esophagus 0.622 (0.318–0.327 ± 0.003, n=7) long by 0.023 (0.019–0.028 ± 0.002, n=7) in maximum width. Didelphic and amphidelphic; vulva located 0.58 (0.47– 0.66 ± 0.069, n=7) from caudal extremity, in the posterior sixth of the body. Vagina vera 0.023 (0.017–0.026 ± 0.003, n=7) long, vestibule 0.073 (0.065–0.078 ± 0.005, n=7) long. Anterior ovejector 0.12 (0.11–0.12 ± 0.003, n=7) long [vestibule, 0.073 (0.065–0.078 ± 0.005, n=7); infundibulum, 0.018 (0.013–0.21 ± 0.004, n=7); sphincter, 0.023 (0.021–0.028 ± 0.003, n=7)]; posterior ovejector 0.025 (0.021–0.032 ± 0.004, n=7) long [vestibule, 0.073 (0.049–0.084 ± 0.016, n=7); infundibulum, 0.022 (0.019–0.026 ± 0.002, n=7); sphincter, 0.025 (0.021–0.032 ± 0.004, n=7)]. Eggs in anterior uterine branch: 17 (12–25 ± 4.6, n=7); eggs in the posterior uterine branch: 8 (6–10 ± 1.64, n=7); embryonated, elliptical 0.037 (0.030–0.049 ± 0.006, n=10) long by 0.022 (0.019–0.023 ± 0.002, n=10) wide, forming a single row in the uterus. Anus without lips, 0.063 (0.052–0.075 ± 0.009, n=7) from posterior end. Body terminating in a sharp spine 0.008 (0.006–0.013 ± 0.002, n=6) long.
Remarks. Up to now, 27 species of the genus Molineus have been described around the world (Durette-Desset et al. 1981; Platt & Pence 1981; Wu & Zhang 1984; Durette-Desset & Pesson 1987; Durette-Desset & Corvione 1988; Durette-Desset et al. 2000a, b, 2001): 12 from the Neotropical, 3 from the Ethiopian, 5 from the Oriental, 4 from the Palearctic, and 3 from the Nearctic Realms. Most of the 27 species are parasites of Mammalia ( Carnivora 22 species, Primates 4), and only one species associated with Squamata has been collected ( M. inexpectatus Durette-Desset, Guerrero & Boyer, 2000 ). A mix of morphological traits is diagnostic for Molineus lotoris n. sp.: 1) presence of a prominent and globular cephalic vesicle; 2) spicules length <0.14 (0.10), with 2 terminal processes and 3) males with 17 synlophe ridges at mid-body region.
The new species described in the present study can be differentiated from 20 of the 27 species contained in the genus by having 2 instead of 3 terminal processes in the spicules. Among species having 3 terminal processes in the spicules, M. inexpectatus , M. inglisi Durette-Desset & Pesson, 1987 , M. midas Durette-Desset & Corvione, 1998 , M. springsmithi Inglis & Ogden, 1965 and M. vexillarius Dunn, 1961 are distinguished from the Mexican species because males of those species have a smaller number of synlophe ridges at mid-body level (14, 10–12, 14, NEW MOLINEUS IN MEXICO Zootaxa 4320 (2) © 2017 Magnolia Press · 393 11–15, vs 17, respectively) ( Dunn 1961; Inglis & Ogden 1965; Durette-Desset & Corvione 1998; Durette-Desset et al. 2000b); in contrast, M. patens ( Dujardin, 1845) Petrov, 1928 and M. genettae Cameron, 1927 are differentiated from M. lotoris because they have a greater number of these structures (40 ridges in both species vs 17 in the new one) ( Travassos 1937; Durette-Desset & Pesson 1987). Three other species can be separated from the new species described herein, because its spicules are longer than those of M. lotoris (0.099–0.115): M. nasuae Lent & Freitas, 1938 (0.119–0.184), M. petrovi Durette-Desset & Pesson, 1987 (0.154–0.175) and M. sichuanensis Wu & Zhang, 1984 (0.132–0.159) ( Petrov 1928; Lent & Freitas 1938; Wu et al. 1984). The Palearctic distribution of M. legerae Durette-Desset & Pesson, 1987 and the Nearctic distribution of M. mustelae Schmidt, 1965 as well as the greater body size of males (about twice the size of the Mexican species, i.e., 5.6–7 and 4.2–6 vs 1.8–2.41, respectively) allows distinguishing these 2 species from the new species described herein ( Schmidt 1965; Durette-Desset & Pesson 1987). Molineus europeaus Zunker, 1929 and M. torulosus ( Molin, 1861) have a larger size of body (8.5 and 7.9–9.7, respectively) and spicules (0.145 and 0.172–0.180, respectively), than M. lotoris (1.8–2.41 and 0.099– 0.115, respectively). In addition, M. europaeus is distributed in the Palearctic Realm, while M. lotoris is a Neotropical species, and M. torulosus parasitizes primates and the new species carnivores ( Zunker 1929; Durette- Desset et al. 2001).
Molineus major Cameron, 1936 , Molineus pardalis Cameron, 1936 and M. planicipitis ( Cameron, 1928) Travassos, 1937 differ from M. lotoris n. sp. by having the dorsal lobe separated from lateral lobes while in the new species, the dorsal lobe is merged with the contour of the bursa. In addition, the size of body of M. major (5–5.5) and M. planicipitis (4.3) is greater than that of M. lotoris n. sp. (1.8–2.41) and spicules and gubernaculum of M. pardalis are smaller (0.075 and 0.037) than in the new species (0.10 and 0.048, respectively) ( Cameron 1936; Travassos, 1937). Finally, the body of males and females of M. barbaris Cameron, 1936 (3.4 and 4.1), M. cati Durette-Desset, Boomker & Malan, 2000 (5.1 and 5.7), and M. samueli Platt & Pence, 1981 (8–11.8 and 12.1– 13.6), is larger than the body of both sexes of M. lotoris (1.8–2.41 and 2.6–3, respectively), and the rays 9 are shorter than the dorsal ray in M. barbaris and M. cati instead of larger as in the new species ( Cameron 1936; Durette-Desset et al. 2000a). In addition, M. samueli differs from M. lotoris by having 52 vs 17 synlophe ridges at mid-body level, respectively ( Platt & Pence 1981).
The remaining 7 species have 2 terminal processes in the spicules as in M. lotoris n. sp.; nonetheless, the length of body and the size of spicules are greater in males of M. brachyurus Costa & Freitas, 1967 (5.8–6.9 and 0.205–0.217) and M. cynictis Leroux, 1933 (5.5 and 0.184) than in the new species (1.8–2.41 and 0.099–0.115); in addition, the dorsal lobe of M. lotoris n. sp. is merged with the lateral lobes, while it is distinct in the 2 former species ( Leroux 1933; Costa & Freitas 1967). Molineus barbatus Chandler, 1942 can be separated from the Mexican species because the body size of males is almost twice that of M. lotoris (4.3–4.7 vs 1.8–2.41, respectively); further, males of M. barbatus have 24–28 ridges in the synlophe ( Chandler 1942) and males of the new species have 17 in mid-body region. The presence of a globular cephalic vesicle in the new species enables differentiation from M. asiaticus Tubangui & Masilugnan, 1937 , M. felineus Cameron, 1923 and M. paraensis Travassos, 1937 , which have a cylindrical cephalic vesicle. In addition, these 3 species have the dorsal lobe distinct from lateral lobes ( Travassos 1937; Tubangui & Masilugnan 1937) and the latter 2 possess a smaller number of ridges in the synlophe than M. lotoris n. sp. (12–14 and 14, vs 17, respectively). Spicules of the new species are complex, with the internal process slender, ending in a globular swelling, while in M. asiaticus the spicules terminate in 2 needlelike processes ( Tubangui & Masilugnan 1937).
Molineus lotoris n. sp. most closely resembles M. elegans ( Travassos, 1921) , particularly because both species have a globular cephalic vesicle. However, M. elegans has a cervical groove (absent in the new species), the body of males as well as spicule length are slightly greater in M. elegans (2.9–3.5 and 0.120–0.134 vs 1.8–2.41 and 0.099–0.115, respectively) and their bursa is trilobed (instead of having lobes merged as in M. lotoris ). In addition, number of synlophe ridges in M. elegans is smaller than in M. lotoris (12 vs 17, respectively) (see Travassos 1937).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Molineus lotoris
| Mata-López, Rosario 2017 |
M. cati
| Durette-Desset, Boomker & Malan 2000 |
M. cati
| Durette-Desset, Boomker & Malan 2000 |
M. samueli
| Platt & Pence 1981 |
M. samueli
| Platt & Pence 1981 |
M. brachyurus
| Costa & Freitas 1967 |
Molineus barbatus
| Chandler 1942 |
M. barbatus
| Chandler 1942 |
M. planicipitis ( Cameron, 1928 )
| Travassos 1937 |
M. planicipitis
| Travassos 1937 |
M. asiaticus
| Tubangui & Masilugnan 1937 |
M. paraensis
| Travassos 1937 |
M. asiaticus
| Tubangui & Masilugnan 1937 |
Molineus major
| Cameron 1936 |
Molineus pardalis
| Cameron 1936 |
M . major
| Cameron 1936 |
M. pardalis
| Cameron 1936 |
M. barbaris
| Cameron 1936 |
M. barbaris
| Cameron 1936 |
M. cynictis
| Leroux 1933 |
M. elegans ( Travassos, 1921 )
| Travassos & Darriba 1929 |
M. elegans
| Travassos & Darriba 1929 |
M. elegans
| Travassos & Darriba 1929 |
M. elegans
| Travassos & Darriba 1929 |
M . felineus
| Cameron 1923 |