Heissiella donguri, Souma & Ishikawa, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4751.2.12 |
|
publication LSID |
lsid:zoobank.org:pub:3A01B1DD-26EC-4B30-BC66-A8BD99C774DB |
|
DOI |
https://doi.org/10.5281/zenodo.3718153 |
|
persistent identifier |
https://treatment.plazi.org/id/B129728D-7A4A-4A94-B2E5-C75D2173EC69 |
|
taxon LSID |
lsid:zoobank.org:act:B129728D-7A4A-4A94-B2E5-C75D2173EC69 |
|
treatment provided by |
Plazi |
|
scientific name |
Heissiella donguri |
| status |
sp. nov. |
Heissiella donguri sp. nov.
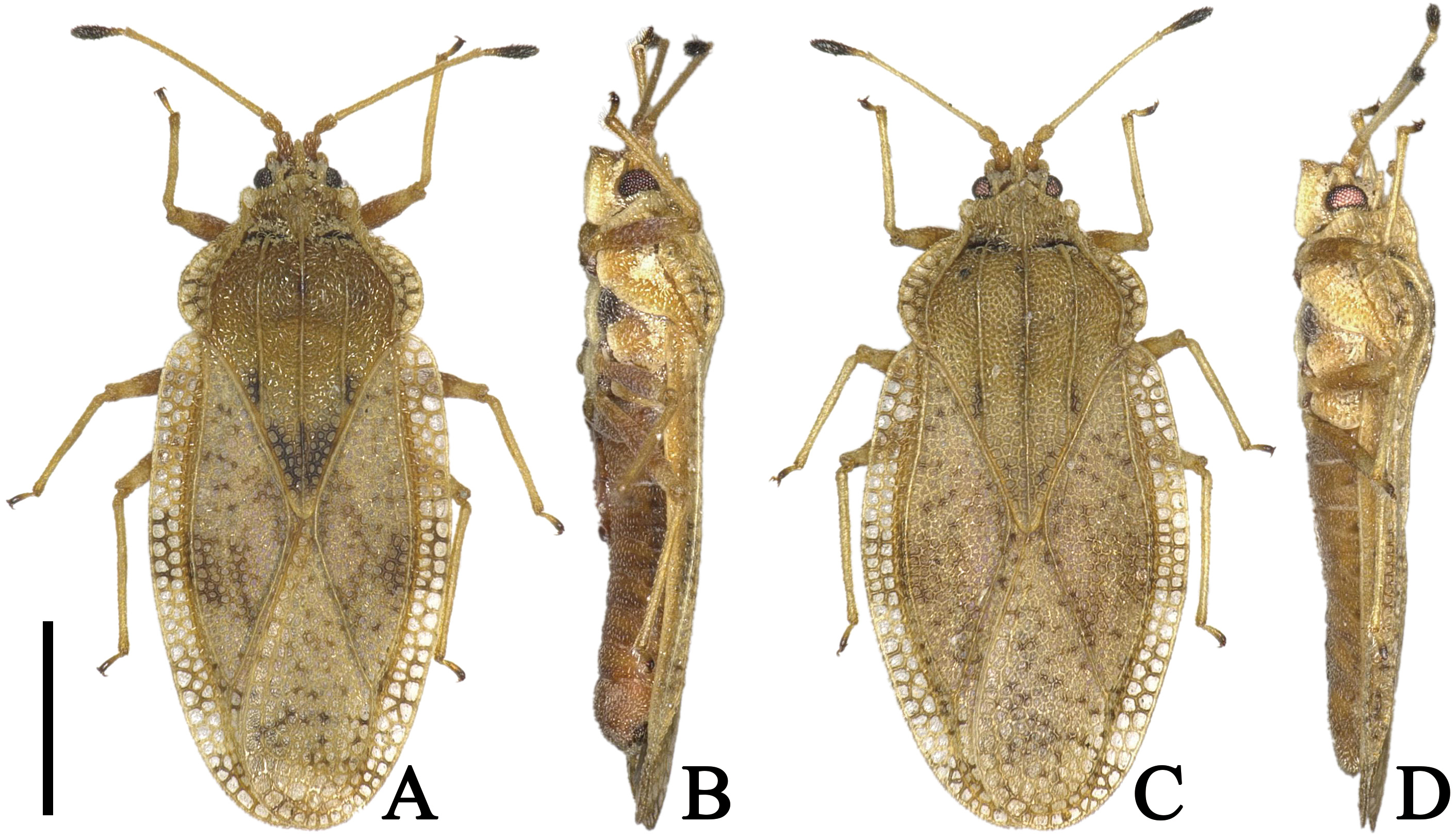
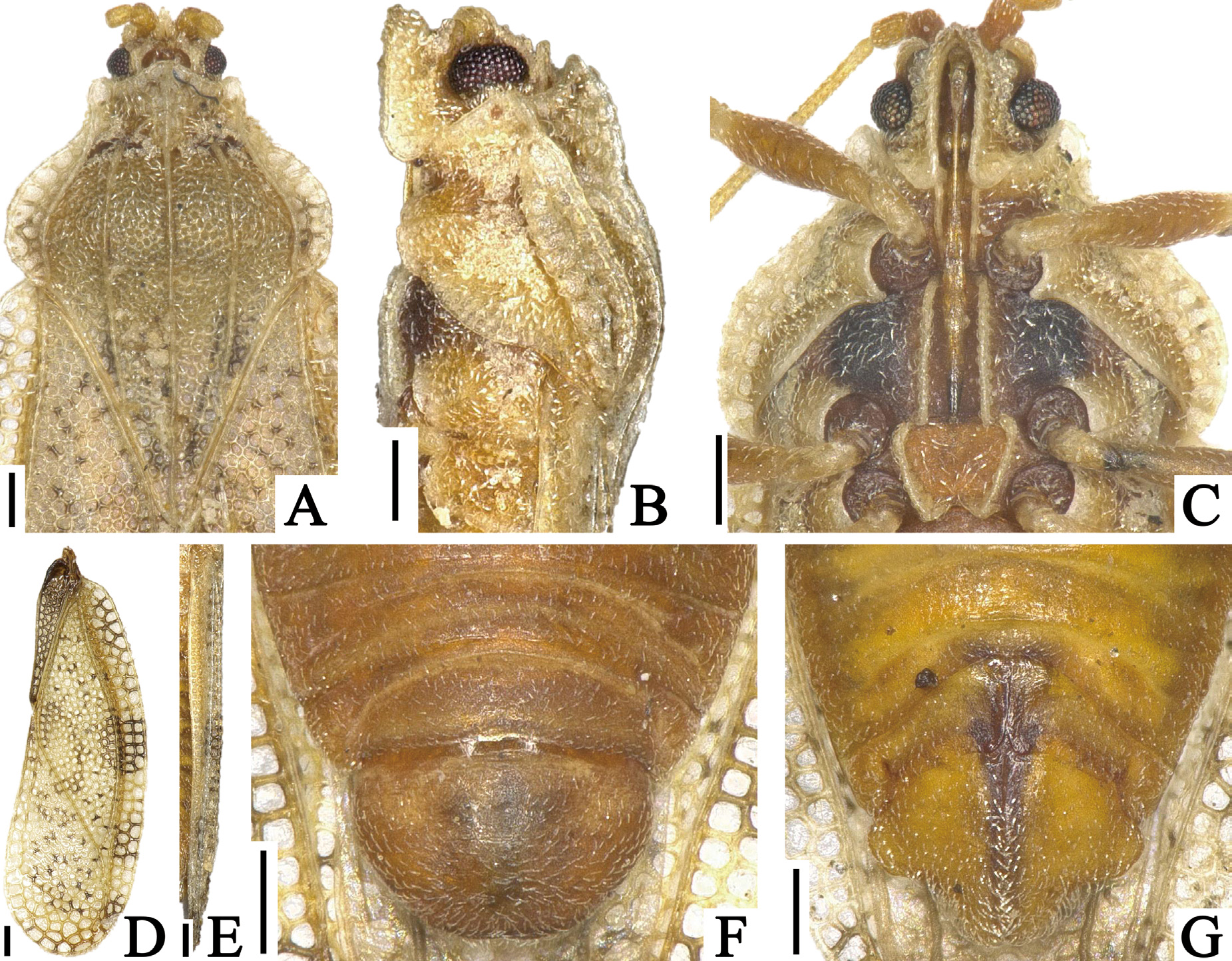
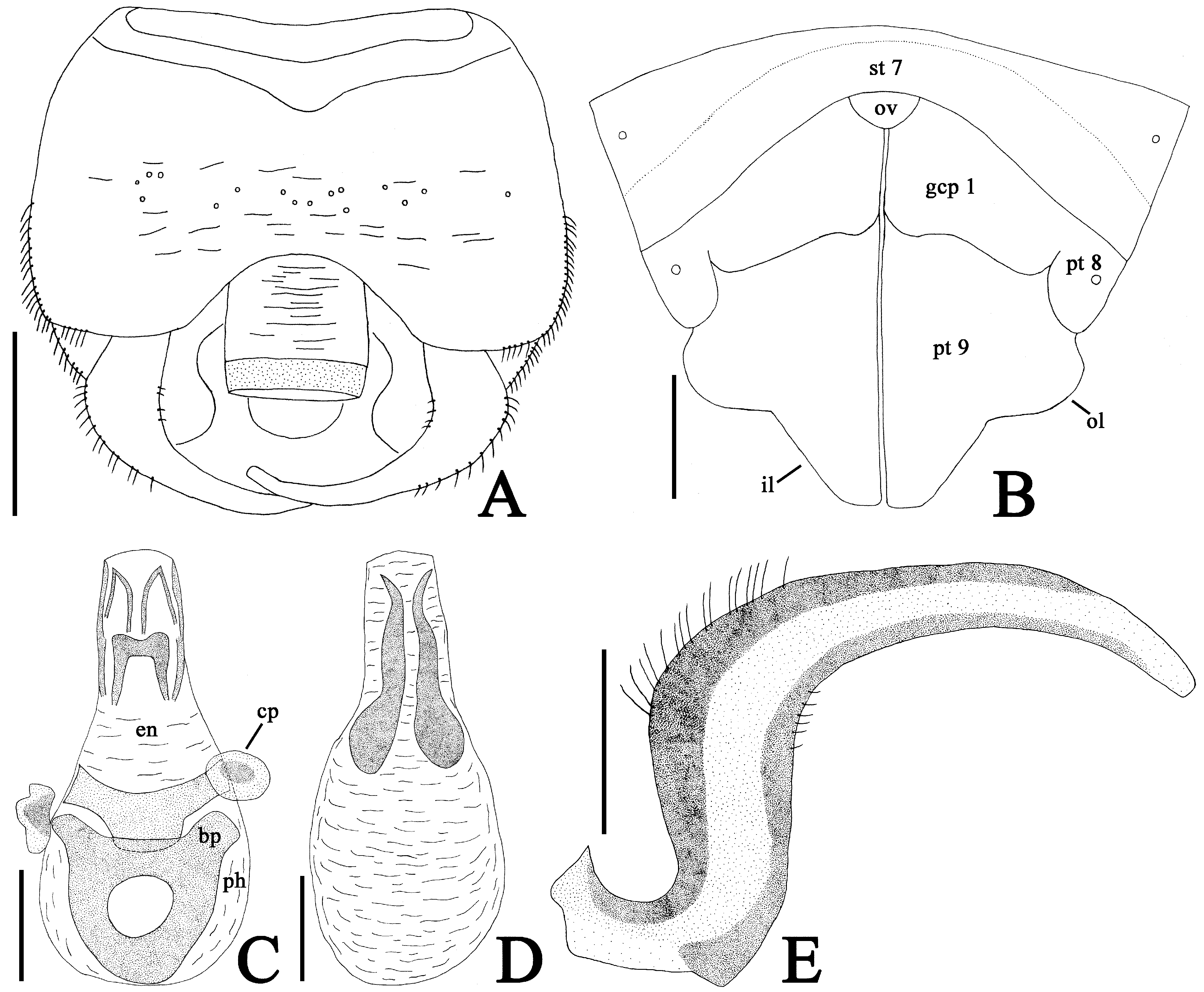
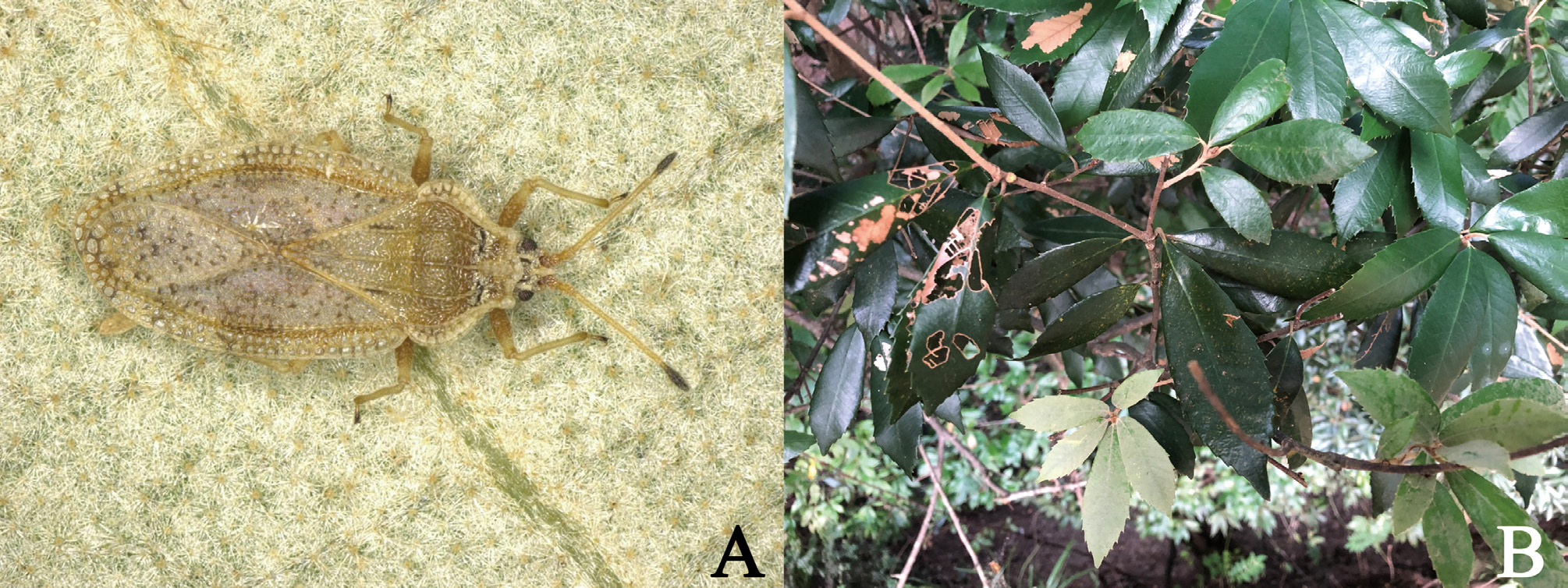
( Figs. 1 View FIGURE 1 A–D, 2A–G, 3A–E, 4A)
Tingis (Tropidocheila) View in CoL sp.: Komatsu (2016: 101) (distribution); Ito (2019: 77) (distribution).
Tingis (Tropidocheila) shaowuana View in CoL (non Drake & Maa, 1953): Miyake (2017: 22) (distribution); Ito & Sasaki (2018: 21) (distribution). Misidentifications.
Type series. HOLOTYPE (macropterous ♂), JAPAN: Honshu : Nara-ken , Nara-shi, Takabatake-cho, 34°40’31.9”N 135°51’07.2”E, 1.x.2019, leg. J. Souma ( TUA) GoogleMaps . PARATYPES (macropterous 41 ♂♂ 27 ♀♀), JAPAN: Honshu: as
holotype (9 ♂♂ 7 ♀♀, TUA; 2 ♂♂ 2 ♀♀, HNHM); as holotype but 14.x.2017, leg. R . Ito (7 ♂♂ 5 ♀♀, TUA); as holotype but 10–11.vi.2017, leg. K. Akita (19 ♂♂ 8 ♀♀, TUA); as holotype but 23.xi.2017, leg. K. Akita (3 ♂♂ 3 ♀♀, TUA) . Kyushu: Oita-ken, Saeki-shi, Ume, Kitakawa Dam, 28.xii.2011, leg. T . Miyake (1 ♀, TUA); Miyazaki Pref., Aya-chô, Kitamata, Kawanaka Shrine, 24.ix.2018, leg. R . Ito (1 ♂ 1 ♀, TUA) .
Diagnosis. Recognized among other species of Heissiella by a combination of the following characters: dorsal outline of hood nearly straight in lateral view ( Fig. 2B View FIGURE 2 ); outer margin of paranotum resting on pronotal disc except anterior end ( Fig. 2A View FIGURE 2 ); costal area of hemelytron with 2–3 rows of areolae throughout its length ( Fig. 2D View FIGURE 2 ); hypocostal lamina with 2 rows of areolae in basal half and a single row in apical half ( Fig. 2E View FIGURE 2 ); endophallus lacking inner sclerite basally ( Fig. 3C, D View FIGURE 3 ); outer lobe of paratergite IX in female protruding laterad, shorter than inner lobe ( Figs. 2G View FIGURE 2 , 3B View FIGURE 3 ); and inner lobe of paratergite IX in female rounded at apex.
Description. Macropterous male. General color brown; compound eyes dark red; calli, antennal segment IV except basal part, thorax in ventral surface, coxa and apex of tarsus black; pubescence on body yellowish ( Figs. 1A, B View FIGURE 1 , 2C View FIGURE 2 ).
Body covered with minute pubescence, 2.3 times as long as maximum width across hemelytra ( Fig. 1A View FIGURE 1 ). Head ( Fig. 2A, B View FIGURE 2 ) smooth; a pair of frontal spines touching each other at apices, reaching beyond tip of tylus; median spine shorter than frontal spines, extending beyond bases of frontal spines; a pair of occipital spines slightly reflexed upward, shorter than median spine, reaching middle part of compound eyes; antenniferous tubercles obtuse, slightly curved inward. Compound eyes prominent laterally, round in dorsal view. Antennae ( Fig. 1A View FIGURE 1 ) smooth; segment I approximately 1.6 times as long as its width; segment II cone-shaped, 1.4 times as long as its maximum width; segment III longest among antennal segments, 1.8 times as long as maximum width of head across compound eyes; segment IV fusiform, widest a little beyond middle, irregularly covered with long pubescence; ratios of lengths from segments I to IV as 1.1: 1.0: 6.9: 2.9. Bucculae long, approximately 2.6 times as long as their maximum height, with 2 rows of areolae throughout its length. Rostrum ( Fig. 2C View FIGURE 2 ) approximately 0.6 times as long as antennae, reaching posterior margin of mesosternum.
Pronotum ( Fig. 2 View FIGURE 2 A–C) 1.4 times as long as maximum width across paranota. Pronotal disc coarsely punctate. Hood obtusely protruded forward, concealing basal fourth of vertex; dorsal outline nearly straight in lateral view. Pronotal carinae with a single row of minute areolae throughout their length; median carina straight, extending to apex of posterior process, higher than hood at maximum height; lateral carinae nearly parallel to each other except anterior end, not concealed by paranota throughout their length, as high as median carina. Calli coarsely punctate. Paranotum widened posteriorly, with 3 rows of areolae at widest part, approximately 4 times as long as its maximum width, slightly covering pronotal disc in posterior part, not touching lateral carinae; outer margin gently curved inward throughout its length. Posterior process triangular, as long as its maximum width.
Hemelytron ( Fig. 2D, E View FIGURE 2 ) narrow, 2.9 times as long as its maximum width, considerably extending beyond apex of abdomen; maximum width across hemelytra 1.2 times as much as maximum width across paranota; clavus with 4 rows of areolae a widest part; costal area wider than subcostal area at widest part, slightly reflexed upward in basal part, with 2–3 rows of areolae throughout its length; subcostal area 0.2 times as wide as discoidal area at middle of hemelytron, with 2 rows of areolae throughout its length; discoidal area distinctly expanding beyond middle of hemelytron, with 10–11 rows of areolae at widest part; sutural area well-developed, with 11–12 rows of areolae at widest part; hypocostal lamina 0.8 times as high as width of costal area at middle of hemelytron, with 2 rows of areolae in basal half and a single row in apical half.
Thoracic pleura coarsely and evenly punctate ( Fig. 2B View FIGURE 2 ). Sternal laminae ( Fig. 2C View FIGURE 2 ) apparently lower than bucculae; pro- and mesosternal laminae nearly parallel to each other, open in both anterior and posterior ends; metasternal laminae as high as mesosternal laminae, open at anterior end, angularly curved inward at posterior end. Legs smooth; femora thickest at middle ( Fig. 1A View FIGURE 1 ).
Abdomen ellipsoidal, 1.5 times as long as its maximum width. Pygophore ( Figs. 2F View FIGURE 2 , 3A View FIGURE 3 ) compressed dorsoventrally, semicircular in ventral view, weakly concave at anterior margin of dorsum, slightly elevated at center of venter, smooth, irregularly punctate in middle part of dorsum. Parameres ( Fig. 3A, E View FIGURE 3 ) thick and long, strongly expanded in middle part, strongly curved inward in apical part; outer and inner margins covered with pubescence in middle part; suspensory arms of parameres completely visible in dorsal view. Phallus ( Fig. 3C, D View FIGURE 3 ) oblong; basal plate and capitate process distinctly sclerotized; phallotheca greatly membranous, with a dorsal sclerite; endophallus membranous except sclerites in its apical part, lacking inner sclerite basally.
Measurements (holotype). Body length with hemelytra 3.6 mm; maximum width across hemelytra 1.6 mm; pronotal width across paranota 1.3 mm.
Macropterous female. General appearance very similar to that of male ( Fig. 1C, D View FIGURE 1 ) except for the following characters: outer lobe of paratergite IX protruding laterad, shorter than inner lobe; inner lobe of paratergite IX rounded at apex ( Fig. 2G View FIGURE 2 , 3B View FIGURE 3 ).
Infraspecific variation among both sexes (holotype and 68 paratypes). Body length with hemelytra 3.3–3.6 mm; maximum width across hemelytra 1.4–1.7 mm; pronotal width across paranota 1.1–1.4 mm.
Brachypterous morph unknown in both sexes.
Remarks. Heissiella species were diagnosed by previous authors based on the differences of different morphological characters, such as shape of the pronotum, arrangement of areolae of the hemelytron, structure of the phallus, and shape of the female paratergite IX ( Péricart 1984; Dang & Bu 2012). These characters are also useful in the identification of the new species.
The structure of the hypocostal lamina of hemelytron being formed by 2 rows of areolae in basal half and a single row in apical half is a feature in which the new species differs from the diagnosis of Heissiella and all members of the closely related genus Trachypeplus ( Dang & Bu 2012; Dang et al. 2013), allowing an easy recognition of H. donguri sp. nov. However, the new species can be provisionally placed into Heissiella based on the paranotum with a longitudinal ridge dividing it into dorsal and ventral parts and the bilobed female paratergite IX.
In the key to all known species of Heissiella ( Dang & Bu 2012) , this new species does not run to any species due to having a combination of the following characters: dorsal outline of hood nearly straight in lateral view ( Fig. 2B View FIGURE 2 ); outer margin of paranotum resting on pronotal disc except anterior end ( Fig. 2A View FIGURE 2 ); costal area of hemelytron with 2–3 rows of areolae throughout its length ( Fig. 2D View FIGURE 2 ); endophallus lacking inner sclerite basally ( Fig. 3C, D View FIGURE 3 ); outer lobe of paratergite IX in female shorter than inner lobe ( Figs. 2G View FIGURE 2 , 3B View FIGURE 3 ); and inner lobe of paratergite IX in female rounded at apex.
Heissiella donguri sp. nov. resembles H. dryadis in the shape of hood and the structure of phallus, but the former is easily distinguished from the latter by the following character states: outer margin of paranotum resting on pronotal disc except anterior end ( Fig. 2A View FIGURE 2 ); costal area of hemelytron with 2–3 rows of areolae throughout its length ( Fig. 2D View FIGURE 2 ); hypocostal lamina with 2 rows of areolae in basal half and a single row in apical half ( Fig. 2E View FIGURE 2 ); and outer lobe of paratergite IX in female protruding laterad ( Figs. 2G View FIGURE 2 , 3B View FIGURE 3 ). In contrast, H. dryadis has the following features: outer margin of paranotum resting on pronotal disc at posterior end; costal area of hemelytron with 2 rows of areolae throughout its length; hypocostal lamina with a single row of areolae throughout its length; and outer lobe of paratergite IX in female protruding posteriad.
The tingid species recorded as ‘ Tingis (Tropidocheila) sp.’ or ‘ Tingis (Tropidocheila) shaowuana Drake & Maa, 1953 ’ by previous authors ( Komatsu 2016; Miyake 2017; Ito & Sasaki 2018; Ito 2019) pertains to the new species.
Distribution. Japan (Honshu, Kyushu) (Fig. 5).
This new species inhabits the laurilignosa ecosystem of Japan proper in a warm-temperate climate and is the first representative of Heissiella from Japan as well as the Palaearctic Region. The discovery of H. donguri sp. nov. represents the easternmost distribution record of Heissiella species.
Etymology. The specific epithet is named after a Japanese common name “donguri” (= acorns of Quercus species), referring to the host plant of the new species; a noun in apposition.
Host plant. Quercus gilva Blume (Fagaceae) ( Fig. 4B View FIGURE 4 ) has been confirmed as a host plant for the new species by several researchers (cf. Miyake 2017; Ito & Sasaki 2018; Ito 2019).
Biology. All the type materials of Heissiella donguri sp. nov. were only collected from the underside of leaves of the host plant Quercus gilva , suggesting that this new species appears to feed on the underside of leaves as do many tingids ( Schuh & Slater 1995).
Adults of the new species were collected in December. Thus, the overwintering form of H. donguri sp. nov. appears to be adult, in accordance with many other tingids in the temperate zone ( Schuh & Slater 1995).
Key to the species of Heissiella View in CoL
The key below is based on a comparison of the type material of the new species with the photographs of the type material of H. dryadis View in CoL (http://n 2t.net/ark:/65665/3e0c902f4- 1126-47 d9-8345-340fe4a365bc), the descriptions and illustrations of other congeners ( Drake & Poor, 1936; Péricart 1984; Dang & Bu 2012), and the available key ( Dang & Bu 2012).
1 Hypocostal lamina of hemelytron with 2 rows of areolae in basal half and a single row in apical half; outer lobe of paratergite IX in female protruding laterad........................................................... H. donguri sp. nov.
- Hypocostal lamina of hemelytron with a single row of areolae throughout its length; outer lobe of paratergite IX in female protruding posteriad................................................................................... 2
2 Outer margin of paranotum resting on pronotal disc except anterior end; outer lobe of paratergite IX in female longer than inner lobe; inner lobe of paratergite IX in female angular at apex............................... H. bicaudata Péricart, 1984 View in CoL
- Outer margin of paranotum resting on pronotal disc at posterior end; outer lobe of paratergite IX in female shorter than inner lobe; inner lobe of paratergite IX in female rounded at apex................................................... 3
3 Dorsal outline of hood arched in lateral view; costal area of hemelytron with 2–3 rows of areolae throughout its length; endophallus with a pair of inner sclerites basally........................................... H. sinica Dang & Bu, 2012
- Dorsal outline of hood nearly straight in lateral view; costal area of hemelytron with 2 rows of areolae throughout its length; endophallus lacking inner sclerite basally........................................ H. dryadis ( Drake & Poor, 1936) View in CoL
FIGURE 5. Distribution of Heissiella donguri sp. nov. in Japan proper. Holotype locality=circle; paratype localities=triangle. Discussion
The Japanese species of Quercus are confirmed as host plants for a number of the phytophagous insects (e.g. Shirozu 2006; Ohbayashi & Niisato 2007; Takizawa 2009). However, only two species belonging to the family Tingidae have been found on Quercus species in Japan ( Yano et al. 2013; Maehara 2014). Therefore, the discovery of Heissiella donguri sp. nov. represents the third record of the Japanese lace bugs feeding on Quercus species and suggests that far more unknown tingids may be newly found on the Japanese oaks with more extensive field research.
This new species feeds on Q. gilva , and a number of individuals of H. dryadis were collected from Q. dilatata Lindl. ex Royle ( Péricart 1984) . Therefore, unrecorded host plants for the remaining two described congeners may be Quercus species as well.
| HNHM |
Hungarian Natural History Museum (Termeszettudomanyi Muzeum) |
| R |
Departamento de Geologia, Universidad de Chile |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Heissiella donguri
| Souma, Jun & Ishikawa, Tadashi 2020 |
Tingis (Tropidocheila) shaowuana
| Ito, R. & Sasaki, S. 2018: 21 |
| Miyake, T. 2017: 22 |
Tingis (Tropidocheila)
| Ito, R. 2019: 77 |
| Komatsu, T. 2016: 101 |