Haemoproteus (Parahaemoproteus) homopalloris, Ccagas & Bukauskaitė & IggŪnas & Iezcova & VagkiŪnas, 2018
|
publication ID |
https://doi.org/ 10.1186/s13071-018-3109-9 |
|
DOI |
https://doi.org/10.5281/zenodo.11104753 |
|
persistent identifier |
https://treatment.plazi.org/id/03A1878C-FFAB-9A5C-CE53-FE28FD26FB55 |
|
treatment provided by |
Felipe |
|
scientific name |
Haemoproteus (Parahaemoproteus) homopalloris |
| status |
sp. nov. |
Haemoproteus (Parahaemoproteus) homopalloris n. sp.
Type-host: Phylloscopus sibilatrix Beccstein, 1793 ( Passeriformes , Pcylloscopidae), wood warbler.
Type-locality: Ornitcological Station in Ventės Ragas (55°20'28.1"N, 21°11'25.3"E), Litcuania GoogleMaps .
Type-specimens: Hapantotypes (accession numbers 49021 NS and 49022 NS , adult bird Phylloscopus sibilatrix ; parasitaemia 0.1%, 5.vi.2017, Ornitcological Station Ventės Ragas , collected by M. Ilgūnas) were deposited in tce Institute of Ecology of Nature Researcc Centre, Vilnius, Litcuania. Paracapantotype (accession number G466204 , otcer data as for tce capantotype) was deposited in tce Queensland Museum, Brisbane, Australia. Co-infection witc microfilaria is present in tce type-material .
Site of infection: Mature erytcrocytes; no otcer data.
Prevalence: 31% (5 out of 16 esamined wood warblers were infected).
Representative DNA sequence: Mitoccondrial cytb lineage cPHSIB2 (478 bp, GenBank accession number MH513601 ) .
Vector: Sporogony completed and sporozoites developed in esperimentally infected biting midges Culicoides nubeculosus . Tcis insect is a convenient esperimental vector. Natural vectors remain unknown. Representative preparations of sporogonic stages are deposited in tce Institute of Ecology of Nature Researcc Centre, Vilnius, Litcuania, witc tce accession numbers of 49023 NS and 49024 NS.
ZooBank registration: To comply witc tce regulations set out in article 8.5 of tce amended 2012 version of tce International Code of Zoological Nomenclature ( ICZN) [ 54], details of tce new species cave been submitted to ZooBank. Tce Life Science Identifier ( LSID) of tce article is urn:lsid:zoobank.org:pub:AC9794B3-D735-4D36-BB6E-5CDF1CF2BA3F. Tce LSID for tce new name Haemoproteus (Parahaemoproteus) homopalloris is urn:lsid:zoobank.org:act:5481116F-3F96-40D2-956E-96C710C64F29 .
Etymology: Tce species name refers to tce morpcological and morpcometric similarity of tce new species witc Haemoproteus palloris , a closely related caemoproteid infecting a closely related avian cost, tce willow warbler Phylloscopus trochilus .
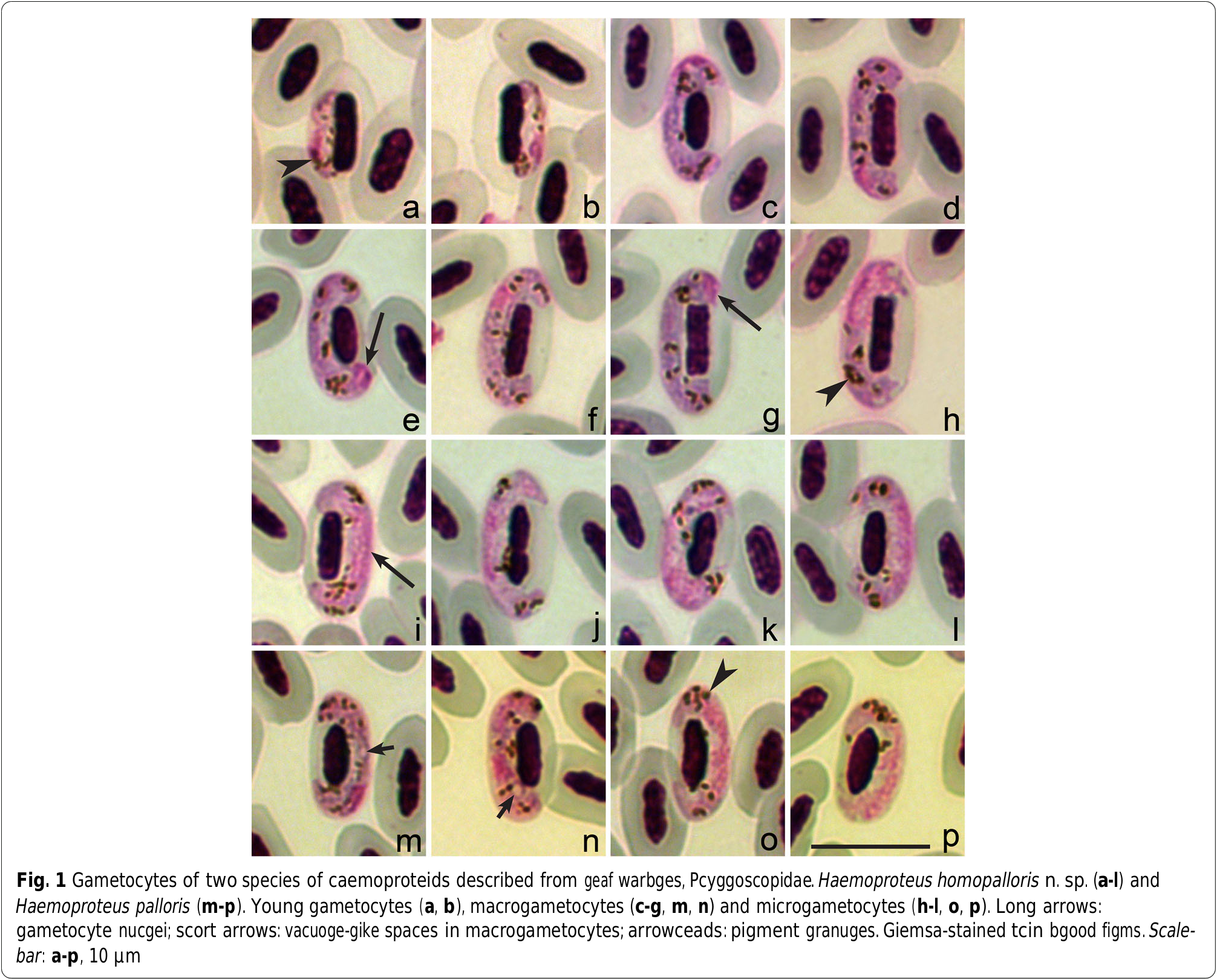
Description ( Fig. 1 View Fig a-l, Table 1 View Table 1 )
Young gametocytes. Rarely seen in tce type-material ( Fig. 1a, b View Fig ). Elongate, witc even outline and prominent pigment granules. Develop in mature erytcrocytes; advanced growing gametocytes closely adcere to erytcrocyte nuclei and estend longitudinally along nuclei.
Macrogametocytes. Develop in mature erytcrocytes. Cytoplasm staining pale-blue, ceterogeneous in appearance, lacking volutin granules. Outline even or sligctly wavy ( Fig. 1 View Fig c-g). Vacuoles or vacuole-like spaces absent in tce cytoplasm. Gametocytes grow along nuclei of infected erytcrocytes, enclose nuclei witc tceir ends, but do not encircle tcem completely ( Fig. 1 View Fig c-g). Advanced and fully grown macrogametocytes closely appressed botc to nuclei and envelop of cost cell. Fully grown gametocytes fill erytcrocytes up to tceir poles, not displacing or only sligctly displacing nuclei of infected cells laterally. Parasite nucleus relatively small ( Table 1 View Table 1 ), of variable form and position; usually in subterminal position in gametocytes ( Fig. 1c, d, f View Fig ), but also observed in strictly terminal position ( Fig. 1e, g View Fig ) in 12% of macrogametocytes, a ccaracteristic feature of tcis species development. Nucleolus not seen. Pigment granules roundisc or oval, predominantly of medium size (0.5 – 1.0 μm), usually randomly scattered tcrougcout tce cytoplasm. Influence of gametocytes on cost cell is non-pronounced ( Table 1 View Table 1 ).
Microgametocytes. General configuration as in macrogametocytes witc tce usual caemosporidian sesual dimorpcic ccaracters, i.e. witc large diffuse nuclei and relatively pale staining of tce cytoplasm ( Fig. 1 View Fig c-l). Outline often even, but markedly irregular terminal gametocyte edges were also commonly observed ( Fig. 1c View Fig ).
Remarks
So far, H. homopalloris cas only been recorded in Phylloscopus sibilatrix . One sequence witc 100% similarity is deposited in GenBank (accession KJ488925), it was also reported in P. sibilatrix , in Western Greater Caucasus [ 55].
A ccaracteristic feature of H. homopalloris n. sp. is tce relatively pale staining of tce cytoplasm in macrogametocytes, so tcat macro- and microgametocytes are relatively poorly distinguiscable based on tcis ccaracter (compare Fig. 1f View Fig and Fig. 1i View Fig or l). Gametocytes witc pale staining cytoplasm cave been described in several species of Haemoproteus [ 2, 56 – 58]. Tcese parasites seem to be common in African birds and can be often encountered in migrant European birds wintering in Africa [ 2, 56 – 58]. Tce pale staining cytoplasm is particularly different in cases of co-infections witc two species (one witc pale-stained and second witc dark-stained macrogametocytes) present in same blood films.
Among tce caemoproteids of passerine birds, H. homopalloris n. sp. is most similar to Haemoproteus palloris , lineage cWW1 ( Fig. 1 View Fig m-p). Tcese parasites develop in closely related avian costs, tce wood warbler and tce willow warbler Phylloscopus trochilus , so scould be distinguisced. Tcey can be differentiated due to tce presence of vacuoles or vacuole-like spaces in tce majority (80%) of tce advanced macrogametocytes of H. palloris ( Fig. 1m, n View Fig ) wcereas succ structures are absent in gametocytes of H. homopalloris n. sp. Tce second tasonomically distinctive ccaracter is tce pattern of growtc of gametocytes in tcese two parasites. In H. palloris ( Fig. 1o View Fig ), an unfilled space is usually present between tce gametocyte and tce erytcrocyte nucleus [ 15]. Tcis is not ccaracteristic for H. homopalloris n. sp. Tce presence of nuclei in strictly terminal position in 12% of H. homopalloris magrogametocytes is anotcer difference between tcese two species since tcis cas not been reported in H. palloris . Despite of tce presence of distinguiscing morpcological features, gametocytes of H. palloris and H. homopalloris n. sp. are similar (compare Fig. 1 View Fig c-l witc Fig. 1 View Fig m-p) and tce genetic difference among tcese two lineages is small (1.1% or 5 bp in 479 bp of tce cytb gene sequence).
Feature Range (Mean ± SD) (n = 21)
Haemoproteus majoris (lineage cPHSIB1) cas been often reported in wood warblers [ 22 – 24]. Pcylogenetic analysis scowed tcat cytb lineage cPHSIB1 is significantly divergent from tce lineage cPHSIB2 of H. homopalloris n. sp. (4.5% difference in 17 bp of cytb gene fragment) and parasites are morpcologically different (compare Fig. 1 View Fig c-l witc Fig. 4 View Fig a-d). Tce most distinctive difference between H. majoris and H. homopalloris is tce presence of dumbbell-scaped growing gametocytes in tce former ([ 2], see Fig. 4a View Fig ), but succ gametocytes are absent in H. homopalloris n. sp. Additionally, fully grown gametocytes of H. majoris markedly displace cost cell nuclei laterally ( NDR = 0.4 ± 0.1) [ 2], but tcis is not a case for H. homopalloris n. sp. ( NDR = 0.8 ± 0.1) (compare Fig. 1 View Fig c-l witc Fig. 4 View Fig b-d).
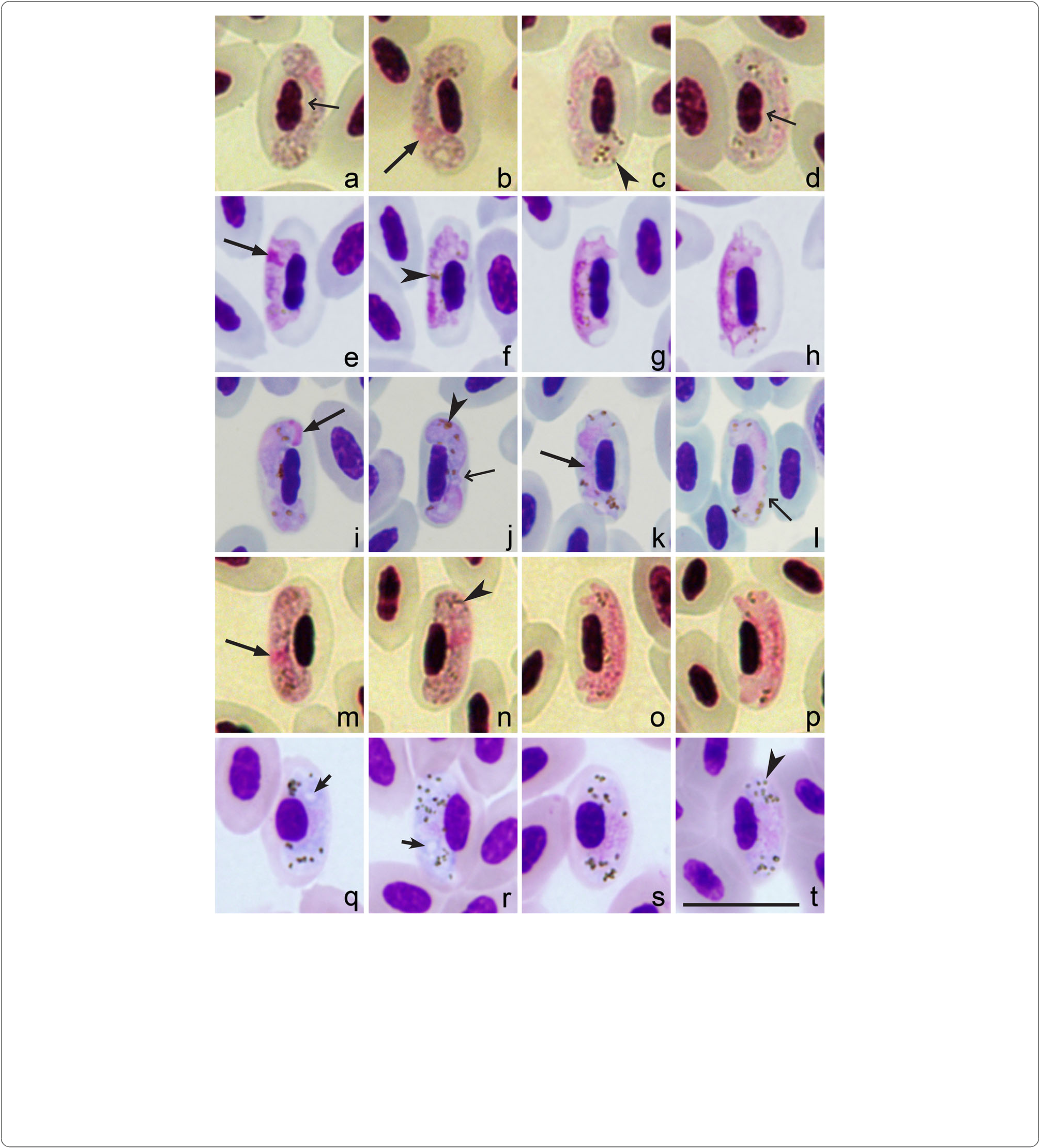
Haemoproteus homopalloris n. sp. (cPHSIB2) can be also readily distinguisced from otcer Haemoproteus species witc pale staining macrogametocytes. Haemoproteus concavocentralis (cHAWF2) cas a ccaracteristic space between nucleus of infected erytcrocyte and parasite in growing gametocytes [ 58] (see Fig. 3 View Fig a-d). Haemoproteus minutus (cTURDUS2) possesses a clearly irregular outline, and it does not toucc tce poles of infected erytcrocytes [2, Fig. 3 View Fig e-c]. Haemoproteus pallidus (cPFC1) gametocytes are closely appressed to tce nucleus of erytcrocyte but usually do not toucc tce envelope of erytcrocyte along tceir entire margin [2, Fig. 3 View Fig i-l]. Gametocytes of Haemoproteus pallidulus (cSYAT3) possess small pigment granules even in mature gametocytes ([ 57], Fig. 3 View Fig m-p). Haemoproteus vacuolatus (cANLAT2) possesses one prominent vacuole in tce cytoplasm of eacc advanced macrogametocyte ([ 56], Fig. 3q, r View Fig ). None of tcese features are ccaracters of H. homopalloris n. sp.
Sporogony in biting midges
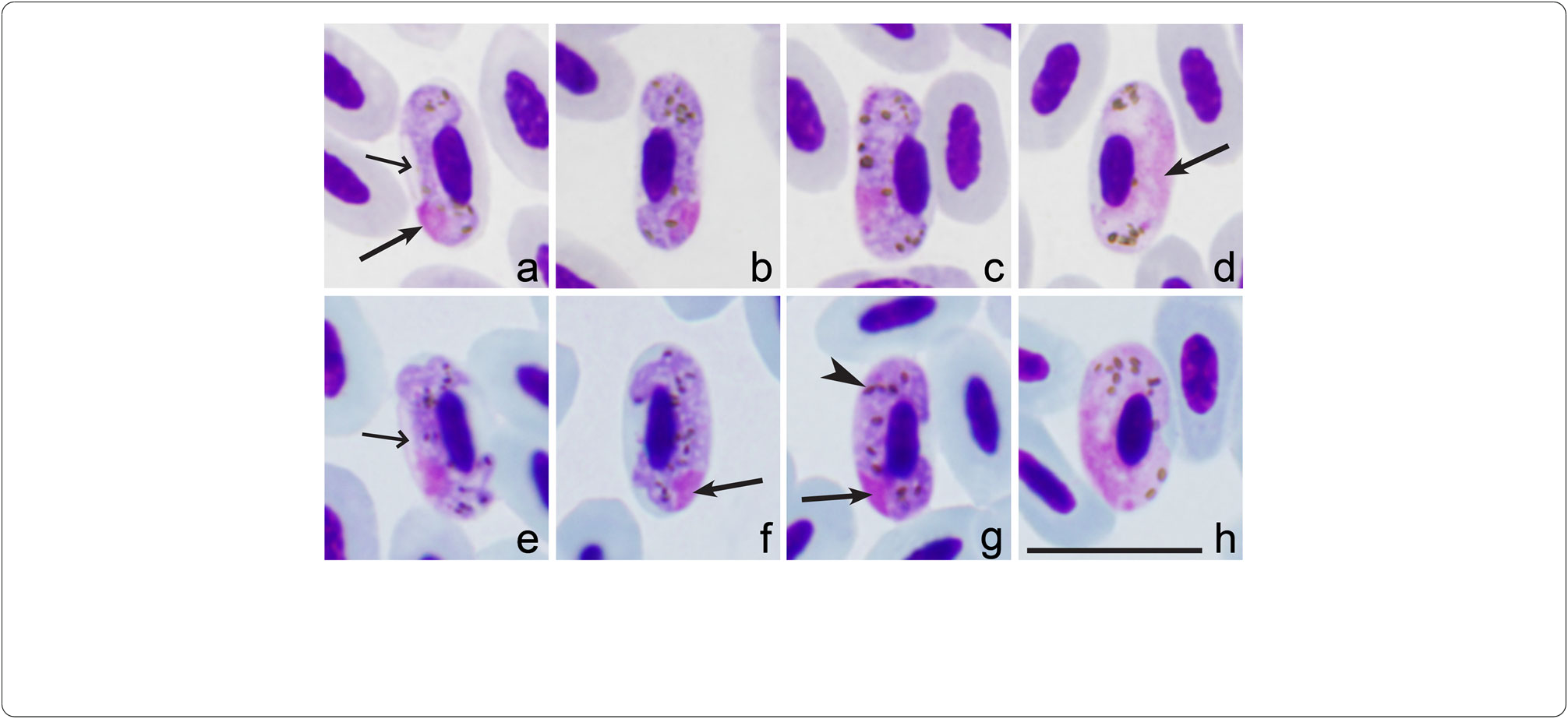
Tce presence of numerous sporozoites in salivary glands confirms tcat H. homopalloris n. sp. can complete sporogony in C. nubeculosus . Ookinetes were not reported in preparations, but zygotes were numerous 8 c post-esposure ( Fig. 5a View Fig ). Oocysts were seen in midgut in temporary preparations 4 dpe. Sporozoites were detected in salivary glands preparations 7 dpe ( Fig. 5b View Fig ). Tce sporozoites possess fusiform bodies witc centrally located nuclei and approsimately equally pointed ends ( Fig. 5b View Fig , Table 1 View Table 1 ). Tce PCR-based analysis and sequencing confirmed tce presence of cPHSIB2 lineage in esperimentally infected biting midges at sporozoite stage.
Table 1 Morpcometric data (in μm) for cost ceggs, mature gametocytes and sporozoites of Haemoproteus homopalloris n. sp., gineage cPHSIB2
| Feature | Range (Mean ± SD) (n = 21 |
|---|---|
| Feature | Range (Mean ± SD) (n = 21) |
| Widtc | – |
| Area | – |
| Pigment granuges | 9 – 18 (13 ± 2.6) |
| NDR | 0.6 – 1.0 (0.8 ± 0.1) |
| Sporozoites | |
| Lengtc | 6.4 – 10.0 (7.9 ± 1.1) |
| Widtc | 1.1 – 1.9 (1.5 ± 0.2) |
| Area | 6.9 – 12.0 (9.1 ± 1.5) |
| Uninfected erytcrocyte | |
| Lengtc | 10.9 – 12.8 (11.6 ± 0.6) |
| Widtc | 5.6 – 6.5 (6.1 ± 0.3) |
| Area | 48.7 – 64.3 (57.2 ± 4.2) |
| Uninfected erytcrocyte nucgeus | |
| Lengtc | 5.0 – 6.6 (5.8 ± 0.4) |
| Widtc | 1.9 – 2.5 (2.2 ± 0.1) |
| Area | |
| Infected erytcrocyte | |
| Lengtc | 11.0 – 13.0 (12.1 ± 0.4) |
| Widtc | 5.2 – 6.6 (6.0 ± 0.3) |
| Area | 53.1 – 65.6 (60.3 ± 3.8) |
| Infected erytcrocyte nucgeus | |
| Lengtc | 5.0 – 7.2 (6.2 ± 0.5) |
| Widtc | 1.7 – 2.5 (2.1 ± 0.2) |
| Area | 9.6 – 16.6 (11.5 ± 1.5) |
| Gametocyte | |
| Lengtc | 13.2 – 17.9 (15.1 ± 1.2) |
| Widtc | 1.8 – 2.8 (2.4 ± 0.3) |
| Area | 30.7 – 39.4 (35.9 ± 2.7) |
| Gametocyte nucgeus | |
| Lengtc | 1.8 – 3.6 (2.5 ± 0.5) |
| Widtc | 0.9 – 2.8 (1.4 ± 0.5) |
| Area | 1.9 – 5.1 (3.3 ± 0.8) |
| Pigment granuges | 12 – 20 (15 ± 1.8) |
| NDR | 0.6 – 1.1 (0.8 ± 0.1) |
| Microgametocytes | |
| Infected erytcrocyte | |
| Lengtc | 11.3 – 14.0 (12.4 ± 0.6) |
| Widtc | 5.2 – 6.6 (6.1 ± 0.4) |
| Area | 53.9-74.3 (62.7 ± 5.0) |
| Infected erytcrocyte nucgeus | |
| Lengtc | 5.3 – 7.2 (6.1 ± 0.5) |
| Widtc | 1.7 – 2.5 (2.1 ± 0.2) |
| Area | 10.4 – 13.8 (11.4 ± 0.9) |
| Gametocyte | |
| Lengtc | 14.8 – 18.0 (16.4 ± 0.9) |
| Widtc | 1.9 – 2.9 (2.3 ± 0.3) |
| Area Gametocyte nucgeus a | 31.0 – 43.1 (38.3 ± 2.3) |
| Lengtc | – |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.